CMS Labelers with Signed Rebate Agreement As of 02 14 18
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

05/01/02 Louisiana Medicaid Management
APPENDIX C 05/01/02 LOUISIANA MEDICAID MANAGEMENT INFORMATION SYSTEM PAGE 1 DEPT OF HEALTH AND HOSPITALS - BUREAU OF HEALTH SERVICES FINANCING LOUISIANA MEDICAID PHARMACY BENEFITS MANAGEMENT UNIT ONLY THESE COMPANIES PRODUCTS ARE COVERED AND ONLY THOSE DOSAGE FORMS LISTED IN APPENDIX A. MEDICAID DRUG FEDERAL REBATE PARTICIPATING PHARMACEUTICAL COMPANIES LABELER PHARMACEUTICAL COMPANY EFFECTIVE END DATE CODE DATE 00002 ELI LILLY & CO 04/01/91 00003 E.R.SQUIBB & SONS,INC 04/01/91 00004 HOFFMAN-LA ROCHE,INC 04/01/91 00005 LEDERLE LABORATORIES AMERICAN CYANAMID 04/01/91 00006 MERCK SHARP & DOHME 04/01/91 00007 SMITHKLINE BEECHAM CORPORATION 04/01/91 00008 WYETH LABORATORIES 04/01/91 00009 THE UPJOHN COMPANY 04/01/91 00011 BECTON DICKINSON MICROBIOLOGY SYSTEMS 10/01/91 07/01/98 00013 ADRIA LABORATORIES DIV.OF ERBAMONT,INC 04/01/91 00014 G.D.SEARLE & CO 04/01/91 01/01/01 00015 MEAD JOHNSON & COMPANY 04/01/91 00016 KABI PHARMACIA 04/01/91 00021 REED & CARNRICK 10/01/96 01/01/97 00023 ALLERGAN,INC 04/01/91 00024 WINTHROP PHARMACEUTICALS 04/01/91 00025 G.D.SEARLE & CO 04/01/91 00026 MILES INC.,PHARMACEUTICAL DIVISION 04/01/91 00028 GEIGY PHARMACEUTICALS 04/01/91 00029 SMITHKLINE BEECHAM CORPORATION 04/01/91 00031 ROBINS,A.H. 04/01/91 00032 SOLVAY PHARMACEUTICALS 04/01/91 00033 SYNTEX 04/01/91 00034 THE PURDUE FREDERICK COMPANY 04/01/91 00037 CARTER-WALLACE,INC 04/01/91 00038 STUART PHARMACEUTICALS,ICI AMERICAS INC 04/01/91 07/01/01 00039 HOECHST-ROUSSEL PHARMACEUTICALS INC 04/01/91 00043 SANDOZ CONSUMER CORPORATION 04/01/91 00044 KNOLL PHARMACEUTICALS -
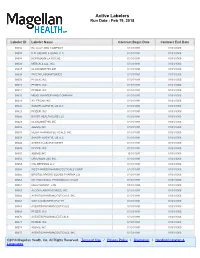
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -
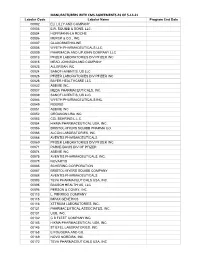
MANUFACTURERS with CMS AGREEMENTS AS of 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY and COMPANY 00003 E.R
MANUFACTURERS WITH CMS AGREEMENTS AS OF 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC Labeler Code Labeler Name Program End Date 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. 00186 ASTRAZENECA PHARMACEUTICALS LP 00187 BAUSCH HEALTH US, LLC. 00206 WYETH PHARMACEUTICALS LLC, 00224 KONSYL PHARMACEUTICALS, INC. 00225 B. F. ASCHER AND COMPANY, INC. 00228 ACTAVIS PHARMA, INC. -

AHRQ Healthcare Horizon Scanning System – Status Update Horizon
AHRQ Healthcare Horizon Scanning System – Status Update Horizon Scanning Status Update: July 2014 Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. HHSA290201000006C Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 July 2014 Statement of Funding and Purpose This report incorporates data collected during implementation of the Agency for Healthcare Research and Quality (AHRQ) Healthcare Horizon Scanning System by ECRI Institute under contract to AHRQ, Rockville, MD (Contract No. HHSA290201000006C). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. A novel intervention may not appear in this report simply because the System has not yet detected it. The list of novel interventions in the Horizon Scanning Status Update Report will change over time as new information is collected. This should not be construed as either endorsements or rejections of specific interventions. As topics are entered into the System, individual target technology reports are developed for those that appear to be closer to diffusion into practice in the United States. A representative from AHRQ served as a Contracting Officer’s Technical Representative and provided input during the implementation of the horizon scanning system. AHRQ did not directly participate in the horizon scanning, assessing the leads or topics, or provide opinions regarding potential impact of interventions. -
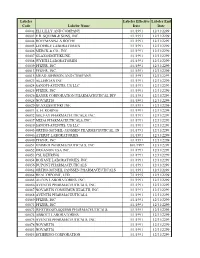
Participating Labelers.Xlsx
Labeler Labeler Effective Labeler End Code Labeler Name Date Date 00002 ELI LILLY AND COMPANY 1/1/1991 12/31/2299 00003 E.R. SQUIBB & SONS, INC. 1/1/1991 12/31/2299 00004 HOFFMANN-LA ROCHE 1/1/1991 12/31/2299 00005 LEDERLE LABORATORIES 1/1/1991 12/31/2299 00006 MERCK & CO., INC. 1/1/1991 12/31/2299 00007 GLAXOSMITHKLINE 1/1/1991 12/31/2299 00008 WYETH LABORATORIES 1/1/1991 12/31/2299 00009 PFIZER, INC 1/1/1991 12/31/2299 00013 PFIZER, INC. 1/1/1991 12/31/2299 00015 MEAD JOHNSON AND COMPANY 1/1/1991 12/31/2299 00023 ALLERGAN INC 1/1/1991 12/31/2299 00024 SANOFI-AVENTIS, US LLC 1/1/1991 12/31/2299 00025 PFIZER, INC. 1/1/1991 12/31/2299 00026 BAYER CORPORATION PHARMACEUTICAL DIV. 1/1/1991 12/31/2299 00028 NOVARTIS 1/1/1991 12/31/2299 00029 GLAXOSMITHKLINE 1/1/1991 12/31/2299 00031 A. H. ROBINS 1/1/1991 12/31/2299 00032 SOLVAY PHARMACEUTICALS, INC. 1/1/1991 12/31/2299 00037 MEDA PHARMACEUTICALS, INC. 1/1/1991 12/31/2299 00039 SANOFI-AVENTIS, US LLC 1/1/1991 12/31/2299 00045 ORTHO-MCNEIL-JANSSEN PHARMECEUTICAL, IN 1/1/1991 12/31/2299 00046 AYERST LABORATORIES 1/1/1991 12/31/2299 00049 PFIZER, INC 1/1/1991 12/31/2299 00051 UNIMED PHARMACEUTICALS, INC 10/1/1997 12/31/2299 00052 ORGANON USA INC. 1/1/1991 12/31/2299 00053 CSL BEHRING 1/1/1991 12/31/2299 00054 ROXANE LABORATORIES, INC. -

05/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 04:25:50 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 05/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 04:25:50 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 07/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -

Manufacturers with Signed Rebate Agreements
Wisconsin Medicaid Pharmacy Data Table Manufacturers with Signed Rebate Agreements January 1, 2013 NEWLABELER NAME START END SC NEW LABELER NAME START END SC 00002 ELI LILLY AND COMPANY 1/1/1991 Y 00131 KREMERS URBAN PHARMACEUTI 1/1/1991 Y 00003 E.R. SQUIBB & SONS, INC. 1/1/1991 Y 00132 C B FLEET COMPANY INC 1/1/1991 00004 HOFFMANN-LA ROCHE 1/1/1991 00135 GLAXOSMITHKLINE 1/1/1995 Y 00005 LEDERLE LABORATORIES 1/1/1991 Y 00143 WEST-WARD PHARMACEUTICAL C 1/1/1991 Y 00006 MERCK & CO., INC. 1/1/1991 Y 00145 STIEFEL LABORATORIES, INC, 1/1/1991 00007 GLAXOSMITHKLINE 1/1/1991 00149 PROCTER & GAMBLE PHARMACE 1/1/1991 00008 WYETH LABORATORIES 1/1/1991 Y 00168 E FOUGERA AND CO. 1/1/1991 Y 00009 PFIZER, INC 1/1/1991 Y 00169 NOVO NORDISK, INC. 1/1/1991 Y 00013 PFIZER, INC. 1/1/1991 Y 00172 IVAX PHARMACEUTICALS, INC. 1/1/1991 Y 00015 MEAD JOHNSON AND COMPANY 1/1/1991 Y 00173 GLAXOSMITHKLINE 1/1/1991 00023 ALLERGAN INC 1/1/1991 Y 00178 MISSION PHARMACAL COMPANY 1/1/1991 Y 00024 SANOFI-AVENTIS, US LLC 1/1/1991 Y 00182 GOLDLINE LABORATORIES, INC. 1/1/1991 Y 00025 PFIZER, INC. 1/1/1991 Y 00185 EON LABS, INC. 1/1/1991 Y 00026 BAYER CORPORATION PHARMAC 1/1/1991 Y 00186 ASTRAZENECA LP 1/1/1991 Y 00029 GLAXOSMITHKLINE 1/1/1991 00187 VALEANT PHARMACEUTICALS NO 1/1/1991 Y 00031 A. H. ROBINS 1/1/1991 Y 00206 LEDERLE PIPERACILLIN 1/1/1991 Y 00032 SOLVAY PHARMACEUTICALS, INC. -
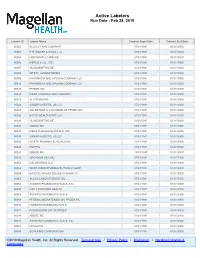
Active Labelers Run Date : Feb 28, 2019
Active Labelers Run Date : Feb 28, 2019 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 07/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 07/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 07/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PHARMACIA AND UPJOHN COMPANY LLC 01/01/1991 01/01/3000 00013 PHARMACIA AND UPJOHN COMPANY LLC 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 07/01/1991 01/01/3000 00023 ALLERGAN INC 07/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 07/01/1991 01/01/3000 00025 GD. SEARLE LLC DIVISION OF PFIZER INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 07/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 07/01/1991 01/01/3000 00046 WYETH PHARMACEUTICALS INC. 01/01/1991 01/01/3000 00049 ROERIG 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 07/01/1991 01/01/3000 00053 CSL BEHRING LLC 07/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 07/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. -
Medicaid System (Mmis) Illinois Department of Run Date: 11/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:25:21 Report Id 2794D052 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 11/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:25:21 REPORT ID 2794D052 PAGE: 01 ALPHA COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 01/01/2017 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 68782 (OSI) EYETECH 46122 AMERISOURCE BERGEN DRUG COMPANY 00074 ABBOTT LABORATORIES 05551 AMGEN INC 68817 ABRAXIS BIOSCIENCE, LLC 55513 AMGEN USA 63090 ACADIA PHARMACEUTICALS, INC. 58406 AMGEN/IMMUNEX 69448 ACCORD BIOPHARMA INC 70121 AMNEAL BIOSCIENCES 16729 ACCORD HEALTHCARE INCORPORATED 53746 AMNEAL PHARMACEUTICALS 42192 ACELLA PHARMACEUTICALS, LLC 65162 AMNEAL PHARMACEUTICALS LLC 10144 ACORDA THERAPEUTICS, INC. 69238 AMNEAL PHARMACEUTICALS, LLC 00472 ACTAVIS 53150 AMNEAL-AGILA, LLC 00228 ACTAVIS ELIZABETH LLC 00548 AMPHASTAR PHARMACEUTICALS, INC. 45963 ACTAVIS INC. 69918 AMRING PHARMACEUTICALS INC. 46987 ACTAVIS KADIAN LLC 66780 AMYLIN PHARMACEUTICALS, INC. 49687 ACTAVIS KADIAN LLC 55724 ANACOR PHARMACEUTICALS 14550 ACTAVIS PHARMA MFGING PRIVATE LIMITED 10370 ANCHEN PHARMACEUTICALS, INC. 61874 ACTAVIS PHARMA, INC. 43595 ANGELINI PHARMA, INC. 67767 ACTAVIS SOUTH ATLANTIC 62559 ANIP ACQUISITION COMPANY 66215 ACTELION PHARMACEUTICALS U.S., INC. 54436 ANTARES PHARMA, INC. 52244 ACTIENT PHARMACEUTICALS 52609 APO-PHARMA USA, INC. 75989 ACTON PHARMACEUTICALS 60505 APOTEX CORP. 69547 ADAPT PHARMA INC. 63323 APP PHARMACEUTICALS, LLC. 76431 AEGERION PHARMACEUTICALS, INC. 43485 APRECIA PHARMACEUTICALS COMPANY 50102 AFAXYS, INC. 42865 APTALIS PHARMA US, INC 10572 AFFORDABLE PHARMACEUTICALS, LLC 58914 APTALIS PHARMA US, INC. 27241 AJANTA PHARMA LIMITED 13310 AR SCIENTIFIC, INC. 17478 AKORN INC 08221 ARBOR PHARM IRELAND LIMITED 24090 AKRIMAX PHARMACEUTICALS LLC 60631 ARBOR PHARMACEUTICALS IRELAND LIMITED 68220 ALAVEN PHARMACEUTICAL, LLC 24338 ARBOR PHARMACEUTICALS, INC. -
02/04/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:10:22 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 02/04/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:10:22 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 04/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, INC. 00149 PROCTER & GAMBLE PHARMACEUTICALS, INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 INWOOD LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 DUPONT PHARMACEUTICALS 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. 00065 ALCON LABORATORIES, INC. -

Cms Labelers with Signed Rebate Agreement As Of
List of Manufacturers with Federal Rebates as of November 20, 2018 Labeler Labeler Name Program Code End Date 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH LABORATORIES 00009 PFIZER, INC 00013 PFIZER, INC. 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER, INC. 00026 BAYER HEALTHCARE LLC 00029 GLAXOSMITHKLINE 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 AYERST LABORATORIES 00049 PFIZER, INC 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING LLC 00054 WEST-WARD PHARMACEUTICALS CORP. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00066 AVENTIS PHARMACEUTICALS, INC. 00067 GSK CONSUMER HEALTH 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER, INC 00071 PFIZER, INC 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00091 UCB, INC 00093 TEVA PHARMACEUTICALS USA, INC. 00095 ECR PHARMACEUTICALS 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00135 GSK CONSUMER HEALTHCARE HOLDINGS (US)LL 00143 WEST-WARD PHARMACEUTICAL CORP 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 1 of 14 Labeler Labeler Name Program Code End Date 00172 IVAX PHARMACEUTICALS, INC. 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00182 GOLDLINE LABORATORIES, INC. 00185 EON LABS, INC. 00186 ASTRAZENECA PHARMACEUTICALS LP 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00206 LEDERLE PIPERACILLIN 00224 KONSYL PHARMACEUTICALS, INC. 00225 B.