Manufacturers with Signed Rebate Agreements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix a 2016 Financial Report Financial Review Pfizer Inc
Appendix A 2016 Financial Report Financial Review Pfizer Inc. and Subsidiary Companies GLOSSARY OF DEFINED TERMS Unless the context requires otherwise, references to “Pfizer,” “the Company,” “we,” “us” or “our” in this 2016 Financial Report (defined below) refer to Pfizer Inc. and its subsidiaries. We also have used several other terms in this 2016 Financial Report, most of which are explained or defined below: 2016 Financial Report This Financial Report for the fiscal year ended December 31, 2016, which was filed as Exhibit 13 to the Annual Report on Form 10-K for the fiscal year ended December 31, 2016 2016 Form 10-K Annual Report on Form 10-K for the fiscal year ended December 31, 2016 AAV Adeno-Associated Virus ABO Accumulated postretirement benefit obligation ACA (Also referred to as U.S. U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Healthcare Legislation) Act. ACIP Advisory Committee on Immunization Practices ALK anaplastic lymphoma kinase Allergan Allergan plc Alliance revenues Revenues from alliance agreements under which we co-promote products discovered or developed by other companies or us AM-Pharma AM-Pharma B.V. Anacor Anacor Pharmaceuticals, Inc. Astellas Astellas Pharma U.S. Inc. ASU Accounting Standards Update ATM-AVI aztreonam-avibactam Bamboo Bamboo Therapeutics, Inc. Baxter Baxter International Inc. BMS Bristol-Myers Squibb Company CDC U.S. Centers for Disease Control and Prevention Cellectis Cellectis SA Celltrion Celltrion Inc. and Celltrion Healthcare, -
![[Docket No. FDA-2017-N-3203] Wyeth Pharmaceutical](https://docslib.b-cdn.net/cover/0756/docket-no-fda-2017-n-3203-wyeth-pharmaceutical-90756.webp)
[Docket No. FDA-2017-N-3203] Wyeth Pharmaceutical
This document is scheduled to be published in the Federal Register on 06/21/2017 and available online at https://federalregister.gov/d/2017-12908, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration [Docket No. FDA-2017-N-3203] Wyeth Pharmaceuticals Inc. et al.; Withdrawal of Approval of 121 New Drug Applications and 161 Abbreviated New Drug Applications AGENCY: Food and Drug Administration, HHS. ACTION: Notice. SUMMARY: The Food and Drug Administration (FDA) is withdrawing approval of 121 new drug applications (NDAs) and 161 abbreviated new drug applications (ANDAs) from multiple applicants. The holders of the applications notified the Agency in writing that the drug products were no longer marketed and requested that the approval of the applications be withdrawn. DATES: The withdrawal is effective on [INSERT DATE 30 DAYS AFTER DATE OF PUBLICATION IN THE FEDERAL REGISTER]. FOR FURTHER INFORMATION CONTACT: Florine P. Purdie, Center for Drug Evaluation and Research, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 51, rm. 6248, Silver Spring, MD 20993-0002, 301-796-3601. SUPPLEMENTARY INFORMATION: The holders of the applications listed in table 1 in this document have informed FDA that these drug products are no longer marketed and have requested that FDA withdraw approval of the applications under the process in § 314.150(c) (21 CFR 314.150(c)). The applicants have also, by their requests, waived their opportunity for a hearing. Withdrawal of approval of an application or abbreviated application under § 314.150(c) is without prejudice to refiling. 2 Table 1 Application No. -

05/01/02 Louisiana Medicaid Management
APPENDIX C 05/01/02 LOUISIANA MEDICAID MANAGEMENT INFORMATION SYSTEM PAGE 1 DEPT OF HEALTH AND HOSPITALS - BUREAU OF HEALTH SERVICES FINANCING LOUISIANA MEDICAID PHARMACY BENEFITS MANAGEMENT UNIT ONLY THESE COMPANIES PRODUCTS ARE COVERED AND ONLY THOSE DOSAGE FORMS LISTED IN APPENDIX A. MEDICAID DRUG FEDERAL REBATE PARTICIPATING PHARMACEUTICAL COMPANIES LABELER PHARMACEUTICAL COMPANY EFFECTIVE END DATE CODE DATE 00002 ELI LILLY & CO 04/01/91 00003 E.R.SQUIBB & SONS,INC 04/01/91 00004 HOFFMAN-LA ROCHE,INC 04/01/91 00005 LEDERLE LABORATORIES AMERICAN CYANAMID 04/01/91 00006 MERCK SHARP & DOHME 04/01/91 00007 SMITHKLINE BEECHAM CORPORATION 04/01/91 00008 WYETH LABORATORIES 04/01/91 00009 THE UPJOHN COMPANY 04/01/91 00011 BECTON DICKINSON MICROBIOLOGY SYSTEMS 10/01/91 07/01/98 00013 ADRIA LABORATORIES DIV.OF ERBAMONT,INC 04/01/91 00014 G.D.SEARLE & CO 04/01/91 01/01/01 00015 MEAD JOHNSON & COMPANY 04/01/91 00016 KABI PHARMACIA 04/01/91 00021 REED & CARNRICK 10/01/96 01/01/97 00023 ALLERGAN,INC 04/01/91 00024 WINTHROP PHARMACEUTICALS 04/01/91 00025 G.D.SEARLE & CO 04/01/91 00026 MILES INC.,PHARMACEUTICAL DIVISION 04/01/91 00028 GEIGY PHARMACEUTICALS 04/01/91 00029 SMITHKLINE BEECHAM CORPORATION 04/01/91 00031 ROBINS,A.H. 04/01/91 00032 SOLVAY PHARMACEUTICALS 04/01/91 00033 SYNTEX 04/01/91 00034 THE PURDUE FREDERICK COMPANY 04/01/91 00037 CARTER-WALLACE,INC 04/01/91 00038 STUART PHARMACEUTICALS,ICI AMERICAS INC 04/01/91 07/01/01 00039 HOECHST-ROUSSEL PHARMACEUTICALS INC 04/01/91 00043 SANDOZ CONSUMER CORPORATION 04/01/91 00044 KNOLL PHARMACEUTICALS -

Chrysler Affiliate Rewards Program
Chrysler Affiliate Rewards Program If you or your spouse work, or have retired from, one of the companies listed below, you may qualify for pricing as low as 1% Below Factory Invoice on a New FIAT! Check the list of companies below to see if your company qualifies.* A F O AAA-- State of Ohio Members Freightliner Of Tampa, Llc Ocala Freightliner ABB, Inc. Freightliner Of Toledo OCE'- North America Abbott Labs Freightliner Of Utah, Llc Ocean Freightliner, Ltd. Abbott, Nicholson, Quilter, Freightliner Trucks So. Florida Inc. O'connor Gmc, Inc. Esshaki & Youngblood PC Freightliner Twin Ports O'connor Truck Sales, Inc. Abercrombie & Fitch Freightmasters Ohio Machinery Company AboveNet Fresenius Medical dba Ohio CAT Abraxis Bioscience Inc. Fresno Truck Center Oklahoma City Freightliner Accor North America FRIENDLY MOTORCARS Oklahoma Farm Bureau Ace Hardware Corporation Fru-Con Construction Corporation Oklahoma Publishing Company, Action Western Star Fujisawa Healthcare Inc. The (OPUBCO) Actelion Pharmaceuticals US, Inc. Ftl And Ws Of Maine Old Dominion Freight Lines Action Couriers Ftl And Wst Of Tifton Oldcastle Inc. ADVANTAGE Health Solutions, Inc. Ftl Stl Wst Of Odessa Omaha Truck Center Inc Advance Publications Ftl Trucks Of South Florida Omni Care Health Plan Aearo Company Ftl, Stl And Wst Of Montgomery One Call Locators Aetna Ftl,Stl, and Western Star Of Dothan One Source Management Inc Affinia Group Fyda Freightliner Cincinnati Oracle Corporation Agar Truck Sales, Inc. Fyda Freightliner Columbus,Inc Organon Pharmaceuticals USA, Inc. AGCO Corporation Fyda Freightliner Pittsburgh Orlando Freightliner AGFA Corporation Fyda Freightliner Youngstown Orlando Freightliner South Aggreko, LLC ORRIN B HAYES, INC. Agricredit Acceptance LLC G Oscient Pharmaceuticals Agrilink Foods OSI Pharmaceuticals Gabrielli Ford Truck Sales AGSTAR Financial Services Otjen, Van Ert, Stangle, Lieb & Weir, S.C. -

1 United States District Court for the Southern District
Case 1:07-cv-12141-PBS Document 18-3 Filed 11/28/07 Page 1 of 167 UNITED STATES DISTRICT COURT FOR THE SOUTHERN DISTRICT OF IOWA, CENTRAL DIVISION THE STATE OF IOWA, Plaintiff, v. ABBOTT LABORATORIES, INC., AGOURON PHARMACEUTICALS, INC., JURY TRIAL REQUESTED ALPHARMA, INC., ALZA CORPORATION, AMGEN, INC., ASTRAZENECA L.P., ASTRAZENECA PHARMACEUTICALS, LP., AVENTIS BEHRING L.L.C., COMPLAINT BARR LABORATORIES, INC., BAXTER INTERNATIONAL, INC., BAXTER HEALTHCARE CORPORATION, BAYER CORPORATION, BAYER PHARMACEUTICALS CORPORATION, BEN VENUE LABORATORIES, INC., BOEHRINGER INGELHEIM CORPORATION, BOEHRINGER INGELHEIM PHARMACEUTICALS, INC., BRISTOL-MYERS SQUIBB COMPANY, CENTOCOR, INC., CHIRON CORPORATION, DERMIK LABORATORIES, INC., DEY, INC., DEY, L.P., ELI LILLY AND COMPANY, EMD, INC., ENDO PHARMACEUTICALS, INC., ETHEX CORPORATION, ETHICON, INC., FOREST LABORATORIES, INC., FOREST PHARMACEUTICALS, INC. GENEVA PHARMACEUTICALS, GLAXOSMITHKLINE, PLC, GLAXOWELLCOME, INC., GREENSTONE, LTD., HOECHEST MARION ROIUSSEL, INC., HOFFMAN-LAROCHE, INC., 1 Case 1:07-cv-12141-PBS Document 18-3 Filed 11/28/07 Page 2 of 167 IMMUNEX CORPORATION, IVAX CORPORATION, IVAX PHARMACEUTICALS, INC., JANSSEN PHARMACEUTICA PRODUCTS, LP, JOHNSON & JOHNSON, KING PHARMACEUTICALS, INC., KING RESEARCH AND DEVELOPMENT, MCNEIL-PPC, INC., MEDIMMUNE, INC., MERCK & CO., INC., MONARCH PHARMACEUTICALS, INC., MYLAN LABORATORIES, INC., MYLAN PHARMACEUTICALS, INC., NOVARTIS PHARMACEUTICALS CORPORATION, NOVOPHARM USA, INC., ONCOLOGY THERAPEUTICS NETWORK CORP., ORTHO-MCNEIL PHARMACEUTICAL, INC., -

References Used in Algorithms for the Treatment of Persons with Crohn’S Disease
REFERENCES USED IN ALGORITHMS FOR THE TREATMENT OF PERSONS WITH CROHN’S DISEASE 1. AA Pharma Inc: Winpred (prednisone). In: CA Product Monograph. Vaughan, ON; 2018. 2. AbbVie Corporation: Humira (adalimumab). In: CA Product Monograph. St Laurent, QC; 2019. 3. AbbVie Inc: Humira (adalimumab). In: US Product Monograph. North Chicago, IL; 2020. 4. Amgen Canada Inc: Avsola (infliximab). In: CA Product Monograph. Mississauga, ON; 2020. 5. Amgen Inc: Amjevita (adalimumab-atto). In: US Product Monograph. Thousand Oaks, CA; 2019. 6. Amgen Inc: Avsola (infliximab-axxq). In: US Product Monograph. Thousand Oaks, CA; 2019. 7. Antares Pharma Inc: Methotrexate. In: FDA Product Monograph. Ewing, NJ; 2019. 8. Apotex Inc: Methotrexate. In: CA Product Monograph. Toronto, ON; 2019. 9. Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, Kito K, Sugimoto M, Andoh A: NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. Journal of gastroenterology 2016, 51(1):22-29. 10. Aspen Pharmacare Canada Inc: Imuran (azathioprine). In: CA Product Monograph. Oakville, ON; 2019. 11. Biogen Canada Inc: Tysabri (natalizumab). In: CA Product Monograph. Mississauga, ON; 2017. 12. Biogen Idec Inc: Tysabri (natalizumab). In: US Product Monograph. Cambridge, MA; 2019. 13. Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ et al: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clinical pharmacology and therapeutics 2015, 98(1):19-24. 14. Boehringer Ingelheim Pharmaceuticals Inc: Cyltezo (adalimumab-adbm). In: US Product Monograph. Ridgefield, CT; 2019. -

Bio/Pharmaceutical Outsourcing Report: November 2016
Volume 21, Number 11, November 2016 Bio/Pharmaceutical Outsourcing Report Part of PharmSource STRATEGIC ADVANTAGE Business Conditions 1 Business Conditions 1 PE-Owned Companies in Line to Change Hands PE-Owned Companies in Line to Change Hands 2 Commercial Dose Manufacturing and Mergers and acquisitions are a hot topic in the contract services industry these Packaging days, and there is a lot of curiosity about which companies are likely to change 2 New Manufacturing Deal, Temporary hands. Of course, any company is a target at any time, especially when the price is Closures and Recall for Patheon right, but insight to likely acquisition candidates can be gained by looking at the list 3 Commercial Dose Manufacturing and Packaging in Brief 3 Clinical Dose Manufacturing and leastof companies four years owned ago are by likelyprivate targets equity in (PE) the nextfirms. year On oraverage, two. PE firms tend to hold Packaging onto their investments for about five years, so companies that were acquired at 3 Marken to be Acquired by UPS The table on page 2 lists 23 CMC services companies that are PE-owned; the list 5 Clinical Dose Manufacturing and was derived from the PharmSource Strategic Advantage database “Search by Packaging in Brief Ownership” feature. It is sorted by the year each company was acquired, and 6 Side Effects: Impacts of Key Events on suggests that at least 13 CMOs and CDMOs are ripe for a transaction in the foresee- CMOs and CROs able future. 7 API — Large Molecule The sale of one company, Marken (Durham, N.C., USA), was announced just this 7 Samsung BioLogics Completes IPO month (see news item, Page 3); another, Capsugel (Morristown, N.J., USA), has been 8 API — Large Molecule in Brief mentioned in the business press as being prepared for an ownership change. -

Comparison of Oral Contraceptives and Non-Oral Alternatives
PL Detail-Document #290305 −This PL Detail-Document gives subscribers additional insight related to the Recommendations published in− PHARMACIST’S LETTER / PRESCRIBER’S LETTER March 2013 Comparison of Oral Contraceptives and Non-Oral Alternatives —More information about the use of contraceptives is available in our PL Detail-Document, Hormonal Contraception— Productsa Manufacturerb Estrogen Progestin LOW-DOSE MONOPHASIC PILLS Aviane-28 Teva EE 20 mcg Levonorgestrel 0.1 mg Falmina Novast Lessina Teva Lutera Actavis Orsythia Qualitest Sronyx Actavis Gildess Fe 1/20 Qualitest EE 20 mcg Norethindrone acetate Junel 1/20 Teva 1 mg Junel Fe 1/20 Teva Loestrin-21 1/20 Warner Chilcott/Teva Loestrin Fe 1/20 Warner Chilcott/Teva Microgestin 1/20 Actavis Microgestin Fe 1/20 Actavis Generess Fe chewable Actavis EE 25 mcg Norethindrone 0.8 mg Altavera Sandoz EE 30 mcg Levonorgestrel 0.15 mg Kurvelo Lupin Levora Actavis Marlissa Glenmark Nordette-28 Duramed/Teva Portia-28 Teva Cryselle-28 Teva EE 30 mcg Norgestrel 0.3 mg Elinest Novast Low-Ogestrel-21 Actavis Low-Ogestrel-28 Actavis Lo/Ovral-28 Wyeth Gildess Fe 1.5/30 Qualitest EE 30 mcg Norethindrone acetate Junel 1.5/30 Teva 1.5 mg Junel Fe 1.5/30 Teva Loestrin 1.5/30-21 Warner Chilcott/Teva Loestrin Fe 1.5/30 Warner Chilcott/Teva Microgestin 1.5/30 Actavis Microgestin Fe 1.5/30 Actavis More. Copyright © 2013 by Therapeutic Research Center 3120 W. March Lane, Stockton, CA 95219 ~ Phone: 209-472-2240 ~ Fax: 209-472-2249 www.PharmacistsLetter.com ~ www.PrescribersLetter.com ~ www.PharmacyTechniciansLetter.com -

11/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:25:21 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 11/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:25:21 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 01/01/2017 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -

Appendix One the CELLTECH CASE STUDY
City Research Online City, University of London Institutional Repository Citation: Mc Namara, P. (2000). Managing the tension between knowledge exploration and exploitation: the case of UK biotechnology. (Unpublished Doctoral thesis, City University London) This is the accepted version of the paper. This version of the publication may differ from the final published version. Permanent repository link: https://openaccess.city.ac.uk/id/eprint/7870/ Link to published version: Copyright: City Research Online aims to make research outputs of City, University of London available to a wider audience. Copyright and Moral Rights remain with the author(s) and/or copyright holders. URLs from City Research Online may be freely distributed and linked to. Reuse: Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. City Research Online: http://openaccess.city.ac.uk/ [email protected] Managing the Tension Between Knowledge Exploration and Exploitation: The Case of UK Biotechnology By Peter Mc Namara Presented in fulfilment of the requirements of the: Degree of Doctor of Philosophy Strategy and International Business City University Business School Department of Strategy and Marketing March 2000 TABLE OF CONTENTS ACKNOWLEDGEMENTS .......................................................................................................................... -
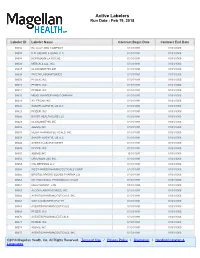
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

SHIRE PLC (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K [X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2009 [ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 0-29630 SHIRE PLC (Exact name of registrant as specified in its charter) Jersey (Channel Islands) 98-0601486 (State or other jurisdiction of incorporation or (I.R.S. Employer Identification No.) organization) 5 Riverwalk, Citywest Business Campus, Dublin +353 1 429 7700 24, Republic of Ireland (Address of principal executive offices and zip code) (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of exchange on which registered American Depositary Shares, each representing three NASDAQ Global Select Market Ordinary Shares 5 pence par value per share Securities registered pursuant to Section 12(g) of the Act: None (Title of class) 1 Indicate by check mark whether the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act Yes [X] No [ ] Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act Yes [ ] No [X] Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.