Customer Rebate List Covering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BIOWORLD TODAY Inquiry
BIOWORLDTM TODAY THE DAILY BIOPHARMACEUTICAL NEWS SOURCE JUNE 30 , 2016 BIOTECH’S MOST RESPECTED NEWS SOURCE FOR MORE THAN 20 YEARS VOLUME 27, NO. 126 LIPID JAM NOT OVER EASILY STOPPED FOR FUTILITY Waffle house? FDA Galena Biopharma implodes as PRESENT review fidgets endpoint, puts cancer vaccine Neuvax future in doubt outcome details; By Jennifer Boggs, Managing Editor Esperion grilled on holdup With the bulk of Galena Biopharma Inc.’s value riding on cancer vaccine Neuvax By Randy Osborne, Staff Writer (nelipepimut-S), the firm’s shares predictably plunged to a new 52-week low Wednesday as an independent data monitoring committee (IDMC) recommended the Analysts had plenty of questions but PRESENT phase III study in breast cancer be stopped for futility following a planned Esperion Therapeutics Inc. offered few interim analysis. But it’s the troubling language in the IDMC’s letter, suggesting the answers regarding the FDA’s stalling on placebo arm might actually have bested the treatment arm, that could signal the end oral, once-daily bempedoic acid (ETC- of the road for Neuvax. 1002) for lipid lowering, after the agency See Galena, page 3 See Esperion, page 4 CHINA DEALS AND M&A IN THE CLINIC Pfizer invests in Asia Merck strikes cancer SUPER ‘NOVA’ with $350M biotech vaccines deal with Tesaro shares blast plant in Hangzhou Moderna, delivering off as niraparib hits By Haky Moon, Staff Writer $200M up front PFS in ovarian cancer HONG KONG – China’s economy may By Michael Fitzhugh, Staff Writer By Marie Powers, News Editor be slowing down, but multinationals Findings from the phase III NOVA trial are positioning themselves to leverage Cancer vaccines tailored to fit tumor- specific profiles are at the heart of a new of niraparib in women with recurrent it as best they can while navigating a ovarian cancer blasted shares of still-complex regulatory environment. -

Fully Human Domain Antibody Therapeutics: the Best of Both Worlds
Drug Discovery Fully Human Domain Antibody Therapeutics: The Best of Both Worlds By combining the therapeutic benefits of small molecule drugs with those of fully human antibodies, Domain Antibodies are expected to have strong therapeutic and commercial potential. By Robert Connelly at Domantis Robert Connelly is Chief Executive Officer of Domantis. He has over 22 years’ commercial experience of the life science sector, including that gained in the fields of diagnostics, drug discovery technologies and antibody therapeutics. Prior to joining Domantis, he was CEO of Veritas Pharmaceuticals (Los Angeles, USA), an in vivo imaging start-up company. He spent over five years with IGEN International, latterly as Senior Vice President and General Manager, Life Sciences, where he took part in the company’s IPO and financing rounds, raising $130 million. The first 11 years of his career were spent at Abbott Laboratories in sales, marketing and management positions. Domain Antibodies (dAbs) are the smallest functional variable regions of either the heavy (VH) or light (VL) binding units of antibodies. At Domantis, we are chains of human antibodies. Domantis scientists applying our proprietary know-how in dAbs to deliver have used the variable domains sequences of human human therapies that address large, unmet medical antibodies to create a series of large and highly needs in areas such as inflammation, cancer and functional libraries of fully human dAbs, with each autoimmune diseases. Three and a half years after library comprising at least 1010 different dAbs. The opening our laboratories, we have a dozen proprietary dAbs selected from these libraries are both specific therapeutic programmes underway, and an additional for their biological target and are well folded and eight therapeutic programmes with partners. -

Infectious Diseases
2013 MEDICINES IN DEVELOPMENT REPORT Infectious Diseases A Report on Diseases Caused by Bacteria, Viruses, Fungi and Parasites PRESENTED BY AMERICA’S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Research Evolves Against Infectious Diseases with Nearly 400 Medicines and Vaccines in Testing Throughout history, infectious diseases hepatitis C that inhibits the enzyme have taken a devastating toll on the lives essential for viral replication. and well-being of people around the • An anti-malarial drug that has shown Medicines in Development world. Caused when pathogens such activity against Plasmodium falci- For Infectious Diseases as bacteria or viruses enter a body and parum malaria which is resistant to multiply, infectious diseases were the current treatments. Application leading cause of death in the United Submitted States until the 1920s. Today, vaccines • A potential new antibiotic to treat methicillin-resistant Staphylococcus Phase III and infectious disease treatments have proven to be effective treatments in aureus (MRSA). Phase II many cases, but infectious diseases still • A novel treatment that works by Phase I pose a very serious threat to patients. blocking the ability of the smallpox Recently, some infectious pathogens, virus to spread to other cells, thus 226 such as pseudomonas bacteria, have preventing it from causing disease. become resistant to available treatments. Infectious diseases may never be fully Diseases once considered conquered, eradicated. However, new knowledge, such as tuberculosis, have reemerged new technologies, and the continuing as a growing health threat. commitment of America’s biopharma- America’s biopharmaceutical research ceutical research companies can help companies are developing 394 medicines meet the continuing—and ever-changing and vaccines to combat the many threats —threat from infectious diseases. -

05/01/02 Louisiana Medicaid Management
APPENDIX C 05/01/02 LOUISIANA MEDICAID MANAGEMENT INFORMATION SYSTEM PAGE 1 DEPT OF HEALTH AND HOSPITALS - BUREAU OF HEALTH SERVICES FINANCING LOUISIANA MEDICAID PHARMACY BENEFITS MANAGEMENT UNIT ONLY THESE COMPANIES PRODUCTS ARE COVERED AND ONLY THOSE DOSAGE FORMS LISTED IN APPENDIX A. MEDICAID DRUG FEDERAL REBATE PARTICIPATING PHARMACEUTICAL COMPANIES LABELER PHARMACEUTICAL COMPANY EFFECTIVE END DATE CODE DATE 00002 ELI LILLY & CO 04/01/91 00003 E.R.SQUIBB & SONS,INC 04/01/91 00004 HOFFMAN-LA ROCHE,INC 04/01/91 00005 LEDERLE LABORATORIES AMERICAN CYANAMID 04/01/91 00006 MERCK SHARP & DOHME 04/01/91 00007 SMITHKLINE BEECHAM CORPORATION 04/01/91 00008 WYETH LABORATORIES 04/01/91 00009 THE UPJOHN COMPANY 04/01/91 00011 BECTON DICKINSON MICROBIOLOGY SYSTEMS 10/01/91 07/01/98 00013 ADRIA LABORATORIES DIV.OF ERBAMONT,INC 04/01/91 00014 G.D.SEARLE & CO 04/01/91 01/01/01 00015 MEAD JOHNSON & COMPANY 04/01/91 00016 KABI PHARMACIA 04/01/91 00021 REED & CARNRICK 10/01/96 01/01/97 00023 ALLERGAN,INC 04/01/91 00024 WINTHROP PHARMACEUTICALS 04/01/91 00025 G.D.SEARLE & CO 04/01/91 00026 MILES INC.,PHARMACEUTICAL DIVISION 04/01/91 00028 GEIGY PHARMACEUTICALS 04/01/91 00029 SMITHKLINE BEECHAM CORPORATION 04/01/91 00031 ROBINS,A.H. 04/01/91 00032 SOLVAY PHARMACEUTICALS 04/01/91 00033 SYNTEX 04/01/91 00034 THE PURDUE FREDERICK COMPANY 04/01/91 00037 CARTER-WALLACE,INC 04/01/91 00038 STUART PHARMACEUTICALS,ICI AMERICAS INC 04/01/91 07/01/01 00039 HOECHST-ROUSSEL PHARMACEUTICALS INC 04/01/91 00043 SANDOZ CONSUMER CORPORATION 04/01/91 00044 KNOLL PHARMACEUTICALS -

2012 Financial Markets Preview Living Creatively
REPRINT FROM JANUARY 2, 2012 BioCentury ™ THE BERNSTEIN REPORT ON BIOBUSINESS Article Reprint • Page 1 of 8 2012 Financial Markets Preview Living creatively By Stacy Lawrence “As the capital markets Viren Mehta of Mehta Partners. “Many Senior Writer companies don’t have any option but to For small, early stage biotech compa- continue to be difficult, raise at lower valuations.” nies, 2012 could be the year of living traditional investors are able He added: “Many companies are not creatively. able to defer any longer. The moment Although public biotechs raised more to extract terms that are comes for many companies when they funds last year than ever before, the vast have to swallow hard and accept painful majority of the money was debt financing more and more onerous.” dilution.” by established companies with marketed products. Thus while the overall numbers Todd Wyche, Brinson Patrick look good and are likely to continue to do Cash on hand so, precommercial companies will have to large and mid-cap companies (see “The Market volatility and macroeconomic get creative with their financings. 1% Effect,” page 2). risk had most buysiders sitting on the Many of these companies avoided rais- In 2011, public biotechs raised $43 sidelines through the back half of 2011. ing money in 2011 because they didn’t billion, easily eclipsing the record $33.1 Bankers are hopeful that this year will be like the valuations, but Wall Streeters say billion in 2000. But debt accounted for more stable, in which case investors might they are now running out of cash. As a 82% of the total dollars raised, compared be willing to support more deal flow dur- result, they will have to use the tricks at to only 19% in 2000 (see “Debt Domi- ing 1H12 (see “Fear Factor,” page 5). -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

DCAT MEMBER COMPANY MEETING LOCATOR V. 4
DCAT MEMBER COMPANY MEETING LOCATOR v. 4 THE BENJAMIN Sancilio Pharmaceuticals Company, Inc. Sri Krishna Pharmaceuticals Ltd. Amino Chemicals Ltd. Zydus Pharmaceuticals (USA) Inc. Apogee Pharma, Inc. C2 PHARMA INTERCONTINENTAL BARCLAY Calyx Chemicals & Pharmaceuticals Ltd. AbbVie* ChemCon GmbH ACIC Pharmaceuticals Inc. Concord Biotech Limited Advitech SA Dipharma Francis Srl Amneal Pharmaceuticals LLC DSM Sinochem Pharmaceuticals ALP Pharm F.I.S. - Fabbrica Italiana Sintetici S.p.A. AMRI Jost Chemical Co. Asymchem Inc. Jubilant Pharma Capsugel, Now a Lonza Company* PharmSource, A GlobalData Company CBC AMERICAS Corp. PolyPeptide Group CellMark USA, LLC ROHNER Inc. Charioteer Pharmaceutical Co., Ltd., Zhejiang FIFTY NYC, A AFFINA HOTEL Chemical and Pharmaceutical Solutions Chiral Quest Corp. Chartwell Pharmaceuticals, LLC Croda, Inc. HOTEL 48LEX DFE Pharma AB BioTechnologies, Inc. DPL-US AiPing Pharmaceutical, Inc. EQ Esteve Almac Evonik Corporation Aptuit LLC FAREVA SA AZAD Fine Chemicals Ltd. Flavine North America, Inc. Cambridge Isotope Laboratories, Inc. Formosa Laboratories, Inc. CMC Biologics Grifols International S.A. Groupe Parima Hainan Poly Pharm. Co., Ltd. Navin Fluorine International Limited Harris Pharmaceutical Qualicaps, Inc. Helm AG RC2 Pharma Connect LLC Hetero USA, Inc. Recipharm Hikal, Ltd. Recro Gainesville LLC Interchem Corporation Reed-Lane, Inc. Inventia Healthcare PVT LTD Please note: Some DCAT member companies have requested not to be listed in the locator. (*) indicates member companies with Business Meeting Spaces in more than one hotel. INTERCONTINENTAL BARCLAY CONT'D PiSA BioPharm, Inc. SPI Pharma Inc. Johnson Matthey Tapemark Kingchem Life Science LLC Unither Pharmaceutical Legacy Pharmaceutical Packaging Uquifa S.A. Lonza AG* Neuland Laboratories Ltd. LOTTE NY PALACE Orion Group AbbVie* Par Pharmaceutical, Inc. -

BTG INTERNATIONAL LIMITED V. AMNEAL PHARMACEUTICALS LLC
United States Court of Appeals for the Federal Circuit ______________________ BTG INTERNATIONAL LIMITED, JANSSEN BIOTECH, INC., JANSSEN ONCOLOGY, INC., JANSSEN RESEARCH & DEVELOPMENT, LLC, Plaintiffs-Appellants v. AMNEAL PHARMACEUTICALS LLC, AMNEAL PHARMACEUTICALS OF NEW YORK, LLC, DR. REDDY'S LABORATORIES, INC., DR. REDDY'S LABORATORIES, LTD., WOCKHARDT BIO AG, WOCKHARDT USA LLC, WOCKHARDT LTD., MYLAN PHARMACEUTICALS INC., MYLAN INC., WEST-WARD PHARMACEUTICALS CORP., NKA HIKMA PHARMACEUTICALS USA INC., HIKMA PHARMACEUTICALS LLC, TEVA PHARMACEUTICALS USA, INC., Defendants-Appellees PAR PHARMACEUTICAL, INC., PAR PHARMACEUTICAL COMPANIES, INC., RISING PHARMACEUTICALS, INC., Defendants ______________________ 2019-1147 ______________________ Appeal from the United States District Court for the District of New Jersey in Nos. 2:15-cv-05909-KM-JBC, 2:16-cv-02449-KM-JBC, 2:17-cv-06435-KM-JBC, Judge Kevin McNulty. 2 BTG INTERNATIONAL LIMITED v. AMNEAL PHARMACEUTICALS LLC - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - BTG INTERNATIONAL LIMITED, JANSSEN BIOTECH, INC., JANSSEN ONCOLOGY, INC., JANSSEN RESEARCH & DEVELOPMENT, LLC, Plaintiffs-Appellants v. AMERIGEN PHARMACEUTICALS, INC., AMERIGEN PHARMACEUTICALS LIMITED, Defendants-Appellees ______________________ 2019-1148 ______________________ Appeal from the United States District Court for the District of New Jersey in No. 2:16-cv-02449-KM-JBC, Judge Kevin McNulty. - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - JANSSEN -
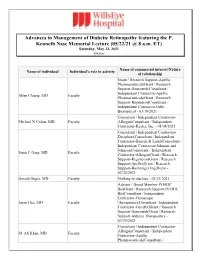
Advances in Management of Diabetic Retinopathy Featuring the P
Advances in Management of Diabetic Retinopathy featuring the P. Kenneth Nase Memorial Lecture (05/22/21 @ 8 a.m. ET) Saturday, May 22, 2021 Online Name of commercial interest/Nature Name of individual Individual's role in activity of relationship Grant / Research Support-Apellis Pharmaceuticals|Grant / Research Support-Genentech|Consultant / Independent Contractor-Apellis Allen Chiang, MD Faculty Pharmaceuticals|Grant / Research Support-Regeneron|Consultant / Independent Contractor-Orbit Biomedical - 03/19/2021 Consultant / Independent Contractor- Michael N Cohen, MD Faculty Allergan|Consultant / Independent Contractor-Keeler, Inc. - 05/04/2021 Consultant / Independent Contractor- Deciphera|Consultant / Independent Contractor-Bausch & Lomb|Consultant / Independent Contractor-Johnson and Johnson|Consultant / Independent Sunir J. Garg, MD Faculty Contractor-Allergan|Grant / Research Support-Regeneron|Grant / Research Support-Apellis|Grant / Research Support-Boehringer Ingelheim - 02/24/2021 Omesh Gupta, MD Faculty Nothing to disclose - 03/21/2021 Advisor / Board Member-IVERIC Bio|Grant / Research Support-IVERIC Bio|Consultant / Independent Contractor-Gyroscope Jason Hsu, MD Faculty Therapeutics|Consultant / Independent Contractor-OccuRx|Grant / Research Support-Genentech|Grant / Research Support-Aldeyra Therapeutics - 03/20/2021 Consultant / Independent Contractor- Allergan|Consultant / Independent M. Ali Khan, MD Faculty Contractor-Apellis Pharmaceuticals|Consultant / Independent Contractor- Genentech|Grant / Research Support- Regeneron -

Amgen Inc. V. Sanofi, No
No. IN THE Supreme Court of the United States ———— AMGEN INC., AMGEN MANUFACTURING LIMITED, AND AMGEN USA, INC., Petitioners, v. SANOFI, AVENTISUB LLC, REGENERON PHARMACEUTICALS INC., AND SANOFI-AVENTIS U.S., LLC, Respondents. ———— On Petition for a Writ of Certiorari to the United States Court of Appeals for the Federal Circuit ———— PETITION FOR A WRIT OF CERTIORARI ———— STUART L. WATT JEFFREY A. LAMKEN WENDY A. WHITEFORD Counsel of Record ERICA S. OLSON MICHAEL G. PATTILLO, JR. EMILY C. JOHNSON SARAH J. NEWMAN AMGEN INC. MOLOLAMKEN LLP One Amgen Center Drive The Watergate, Suite 660 Thousand Oaks, CA 91320 600 New Hampshire Ave., NW (805) 447-1000 Washington, D.C. 20037 (202) 556-2000 [email protected] Counsel for Petitioners :,/621(3(635,17,1*&2,1&± ±:$6+,1*721'& QUESTION PRESENTED The 1952 Patent Act requires patents to “contain a written description of the invention, and of the manner and process of making and using it.” 35 U.S.C. §112(a). The “written description” must be “in such full, clear, concise, and exact terms as to enable any person skilled in the art to which it pertains, or with which it is most nearly connected, to make and use the same.” Ibid. “The object of the statute is to require the patentee to describe his invention so that others may construct and use it after the expiration of the patent.” Schriber-Schroth Co. v. Cleveland Tr. Co., 305 U.S. 47, 57 (1938). The Federal Circuit has construed §112(a) as impos- ing separate “written description” and “enablement” re- quirements subject to different standards. -

Monday, April 22 Chicago Bears Room Chicago Bulls Room Chicago Cubs Room Merck KLOX Technologies Immune Design Leading Biote
As of 4/23/2013 Schedule subject to change Monday, Chicago Bears Room Chicago Bulls Room Chicago Cubs Room April 22 Merck KLOX Technologies Immune Design 1:00 PM Leading Biotech/Big Pharma Medical Devices Vaccines Eli Lilly NewSouth Innovations Syntiron 1:15 PM Leading Biotech/Big Pharma University/Academia Vaccines Amgen Radius Health BioCrea 1:30 PM Leading Biotech/Big Pharma Musculoskeletal Neurology/CNS Nat. Inst. of Neurological Dis. & Stroke Cytokinetics Xenon Pharmaceuticals 1:45 PM Neurology/CNS Musculoskeletal Neurology/CNS Curis OrgaNext Research BV Trigemina 2:00 PM Oncology Regenerative Medicine Neurology/CNS Verastem Flexion Therapeutics Neurocrine Biosciences 2:15 PM Oncology Musculoskeletal Hormone Therapy/CNS Michael J. Fox Foundation Antisense Pharma GmbH Versartis 2:30 PM Non-profit/Patient Advocacy Oncology Hormone Therapy Takeda Pharmaceutical Company TBD KODE Biotech 2:45 PM Leading Biotech/Big Pharma Drug Delivery Resverlogix Corp. Advaxis Q Chip 3:00 PM Cardiovascular Disease Oncology Drug Delivery Grünenthal GmbH Array BioPharma 3:15 PM Neurology/CNS Oncology/Drug Discovery Discovery Labs Mersana Therapeutics 3:30 PM Drug Delivery/Pulmonary Oncology Bayer HealthCare Igenica 3:45 PM Leading Biotech/Big Pharma Oncology Presentations are open to all Convention attendees and are located outside the main entrance of the BIO Business Forum As of 4/23/2013 - Schedule subject to change Tuesday, Chicago Bears Room Chicago Bulls Room Chicago Cubs Room Chicago Blackhawks Room April 23 Pfizer 8:00 AM Leading Biotech/Big Pharma -

HEALTH BENEFITS Providing Value for Our Employees
HEALTH BENEFITS Providing Value for Our Employees March 2017 Business Roundtable CEO members lead companies with more than $6 trillion in annual revenues and nearly 15 million employees. The combined market capitalization of Business Roundtable member companies is the equivalent of nearly one-quarter of total U.S. stock market capitalization, and Business Roundtable members invest $103 billion annually in research and development — equal to 30 percent of U.S. private R&D spending. Our companies pay $226 billion in dividends to shareholders and generate $412 billion in revenues for small and medium-sized businesses annually. Business Roundtable companies also make more than $7 billion a year in charitable contributions. Please visit us at www.brt.org, check us out on Facebook and LinkedIn, and follow us on Twitter. Copyright © 2017 by Business Roundtable HEALTH BENEFITS Providing Value for Our Employees March 2017 March 2017 DEAR BUSINESS LEADERS AND STAKEHOLDERS: I am pleased to share with you a new report highlighting what leading U.S. companies are doing to improve health outcomes for our employees and their families. America’s employer-sponsored health benefits system is the foundation of health care in the United States. Of the 209 million Americans who are covered by health insurance plans, 85 percent — or 177 million Americans* — receive health coverage through an employer. This report demonstrates many different ways employers are helping to drive a health care system that delivers better health care quality and value. I hope you find this report helpful. Sincerely, Brian Moynihan Chairman and Chief Executive Officer Bank of America * Bureau of Labor Statistics, Health Insurance Coverage in the United States: 2015, Current Population Reports, September 2016.