Participating Labelers.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcept Pharmaceuticals, Inc
Table of Contents SCHEDULE 14A (RULE 14A 101) INFORMATION REQUIRED IN PROXY STATEMENT SCHEDULE 14A INFORMATION PROXY STATEMENT PURSUANT TO SECTION 14(A) OF THE SECURITIES EXCHANGE ACT OF 1934 (AMENDMENT NO. ) Filed by the Registrant x Filed by a Party other than the Registrant ¨ Check the appropriate box: ¨ Preliminary Proxy Statement ¨ Confidential, for Use of the Commission Only (as permitted by x Definitive Proxy Statement Rule 14a-6(e)(2)) ¨ Definitive Additional Materials ¨ Soliciting Material Pursuant to §240.14a-12 Transcept Pharmaceuticals, Inc. (Name of Registrant as Specified in its Charter) (Name of Person(s) Filing Proxy Statement, if other than the Registrant) Payment of Filing Fee (Check the appropriate box): x No fee required. ¨ Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. (1) Title of each class of securities to which transaction applies: (2) Aggregate number of securities to which transaction applies: (3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): (4) Proposed maximum aggregate value of transaction: (5) Total fee paid: ¨ Fee paid previously with preliminary materials. ¨ Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. (1) Amount Previously Paid: (2) Form, Schedule or Registration Statement No.: (3) Filing Party: (4) Date Filed: Table of Contents NOTICE OF ANNUAL MEETING OF STOCKHOLDERS JUNE 23, 2011 To Our Stockholders: NOTICE IS HEREBY GIVEN that the Annual Meeting of Stockholders of Transcept Pharmaceuticals, Inc., a Delaware corporation, will be held on Thursday, June 23, 2011, at 8:30 a.m., local time, at our office located at 1003 West Cutting Blvd., Suite 110, Point Richmond, California 94804, for the following purposes: 1. -

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

05/01/02 Louisiana Medicaid Management
APPENDIX C 05/01/02 LOUISIANA MEDICAID MANAGEMENT INFORMATION SYSTEM PAGE 1 DEPT OF HEALTH AND HOSPITALS - BUREAU OF HEALTH SERVICES FINANCING LOUISIANA MEDICAID PHARMACY BENEFITS MANAGEMENT UNIT ONLY THESE COMPANIES PRODUCTS ARE COVERED AND ONLY THOSE DOSAGE FORMS LISTED IN APPENDIX A. MEDICAID DRUG FEDERAL REBATE PARTICIPATING PHARMACEUTICAL COMPANIES LABELER PHARMACEUTICAL COMPANY EFFECTIVE END DATE CODE DATE 00002 ELI LILLY & CO 04/01/91 00003 E.R.SQUIBB & SONS,INC 04/01/91 00004 HOFFMAN-LA ROCHE,INC 04/01/91 00005 LEDERLE LABORATORIES AMERICAN CYANAMID 04/01/91 00006 MERCK SHARP & DOHME 04/01/91 00007 SMITHKLINE BEECHAM CORPORATION 04/01/91 00008 WYETH LABORATORIES 04/01/91 00009 THE UPJOHN COMPANY 04/01/91 00011 BECTON DICKINSON MICROBIOLOGY SYSTEMS 10/01/91 07/01/98 00013 ADRIA LABORATORIES DIV.OF ERBAMONT,INC 04/01/91 00014 G.D.SEARLE & CO 04/01/91 01/01/01 00015 MEAD JOHNSON & COMPANY 04/01/91 00016 KABI PHARMACIA 04/01/91 00021 REED & CARNRICK 10/01/96 01/01/97 00023 ALLERGAN,INC 04/01/91 00024 WINTHROP PHARMACEUTICALS 04/01/91 00025 G.D.SEARLE & CO 04/01/91 00026 MILES INC.,PHARMACEUTICAL DIVISION 04/01/91 00028 GEIGY PHARMACEUTICALS 04/01/91 00029 SMITHKLINE BEECHAM CORPORATION 04/01/91 00031 ROBINS,A.H. 04/01/91 00032 SOLVAY PHARMACEUTICALS 04/01/91 00033 SYNTEX 04/01/91 00034 THE PURDUE FREDERICK COMPANY 04/01/91 00037 CARTER-WALLACE,INC 04/01/91 00038 STUART PHARMACEUTICALS,ICI AMERICAS INC 04/01/91 07/01/01 00039 HOECHST-ROUSSEL PHARMACEUTICALS INC 04/01/91 00043 SANDOZ CONSUMER CORPORATION 04/01/91 00044 KNOLL PHARMACEUTICALS -

Pfizer's Bourla
No. 3985 December 13, 2019 line success and business development. “In the next two years, we need to see how the pipeline is delivering,” he said. Bourla has been outspoken that when it comes to business development, he doesn’t see a mega-merger on the hori- zon. Instead, he said he is looking to bring in mid-stage clinical development assets to complement the internal pipeline. It sounds like investors can expect the company to be active on the business development front within those guard- rails. “I want to double it,” he said of the pipeline, which includes 92 projects right now. “And, we are going to double it by bringing in a lot of innovation to comple- ment what we distribute.” The company is focusing business de- velopment on six core therapeutic areas Pfizer’s Bourla: “I Think We Forgot as well, but Bourla indicated the company will be actively building out those areas both through internal investment and What It Looks Like To Grow” external collaboration. “We’re going to be JESSICA MERRILL [email protected] active because Pfizer is a very big plane and it cannot fly with one engine,” he said. fizer Inc. CEO Albert Bourla took in July it will merge the Upjohn business Bourla highlighted Pfizer’s recent acqui- over the top leadership spot from with Mylan NV to form a new generic drug sition of the cancer specialist Array Bio- PIan Read a year ago, but has quickly company to be called Viatris GMBH. Pharma for $11.4bn as an example of the executed on big changes poised to make The resulting Pfizer will be significantly kinds of deals the company will be pursu- Pfizer significantly smaller and faster smaller, with a 2020 annual revenue base ing. -

Health Industry Business Communications Council
Health Industry Business Communications Council Registered Labelers: Accredited Auto-ID Labeling Standards Argentina New MedTek Devices Pty Ltd Oxavita SRL Norseld Pty Ltd. Novadien Healthcare Pty Ltd The following companies Odontit S.A. (and/or their subsidiaries/ PAMPAMED S.R.L. Numedico Technologies Pty Ltd divisions) have applied PATEJIM SRL Opto Global Pty. Ltd. for a Labeler Identification Orthocell Limited Code (LIC) assignment with Austria Prolotus Technologies Pty Ltd HIBCC*. By doing so, they afreeze GmbH Red Milawa Pty Ltd dba Magic Mobility have demonstrated their AMI GmbH SDI Limited commitment to patient safety Bender Medsystems GmbH Signostics Ltd. and logistical efficiency for BHS Technologies GmbH Sirtex Medical Pty Ltd their customers, the industry Metasys Medizintechnik GmbH Smith & Nephew Surgical Pty. Ltd. and the public at large. PAA Laboratories GmbH Staminalift International Limited Safersonic Medizinprodukte Handels The Pipette Company Pty. Ltd. Any organization that is GmbH Thermo Electron Corporation interested in using the HIBC W & H Dentalwerk Burmoos GmbH Vush Pty Ltd uniform labeling system may apply for the assignment of VUSH STIMULATION Australia one or more LICs. William A Cook Australia Pty. Ltd. Adv. Surgical Design & Manufacture, Ltd. Last updated 9-21-2021 AirPhysio Pty Ltd Belgium Annalise-AI Pty Ltd 3M Europe Apollo Medical Imaging Technology Pty Advanced Medical Diagnostics SA/NV Ltd Analis SA/NV Benra Pty Ltd dba Gelflex Laboratories Baxter World Trade Bioclone Australia Pty. Ltd. Bio-Rad RSL Candelis, Inc. Bio-Rad Lab Inc Clinical Diag. Group DePuy Australia Pty. Ltd. Biosource Europe SA For more information, please dorsaVi Ltd Cilag NV contact the HIBCC office at: EC Certification Service GmbH Coris Bioconcept Fink Engineering Pty Ltd Fuji Hunt Photographic Chemicals NV 2525 E. -

CONCERT PHARMACEUTICALS, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 SCHEDULE 14A Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934 Filed by the Registrant x Filed by a Party other than the Registrant ¨ Check the appropriate box: ¨ Preliminary Proxy Statement ¨ Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) x Definitive Proxy Statement ¨ Definitive Additional Materials ¨ Soliciting Material under §240.14a-12 CONCERT PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) (Name of Person(s) Filing Proxy Statement, if other than the Registrant) Payment of Filing Fee (Check the appropriate box): x No fee required. ¨ Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. (1) Title of each class of securities to which transaction applies: (2) Aggregate number of securities to which transaction applies: (3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): (4) Proposed maximum aggregate value of transaction: (5) Total fee paid: ¨ Fee paid previously with preliminary materials. ¨ Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. (1) Amount Previously Paid: (2) Form, Schedule or Registration Statement No.: (3) Filing Party: (4) Date Filed: 1 Concert Pharmaceuticals, Inc. -

The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry
The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry By: Maitri Punjabi Honors Thesis Economics Department The University of North Carolina at Chapel Hill March 2016 Approved: ______________________________ Dr. Jonathan Williams Punjabi 2 Abstract This paper analyzes the effect of the changing nature of innovation on pharmaceutical firm market value from the years 1987 to 2010 by using U.S. patent and claim data. Over the years, firms have started shifting focus from primary innovation to secondary innovation as new ideas and new compounds become more difficult to generate. In this study, we analyze the impact of this patent portfolio shift on the market capitalization of pharmaceutical firms. After using firm fixed effects and the instrumental variable approach, we find that there exists a strong positive relationship between secondary innovations and the market value of the firm– in fact, we find a stronger relationship than is observed between primary innovation and market value. When focusing on the different levels of innovation within the industry, we find that this relationship is stronger for less-innovative firms (those that have produced fewer patents) than it is for highly- innovative firms. We also find that this relationship is stronger for firms that spend less on research and development, complementing earlier findings that research productivity is declining over time. Punjabi 3 Acknowledgements I would primarily like to thank my adviser, Dr. Jonathan Williams, for his patience and constant support. Without his kind and helpful attitude, this project would have been a much more frustrating process. Through his knowledge of the industry, I have gained valuable insight and have learned a great deal about a unique and growing field. -

Management Liability Focus Johnson & Johnson 1
Insured Profile Report – Management Liability Focus Johnson_________________________________________________________________________ & Johnson Company Profile Credit Details Location 1 Johnson and Johnson Plz Overall Credit Risk High Risk New Brunswick, NJ www.jnj.com Number of Legal Derogatory 84 Company Type Public Items Liability Amount $322,285.00 Formerly Known As N/A Experian Intelliscore 2.57 SIC Code 2834 SIC Code Description Pharmaceutical Preparations Experian Intelliscore Percentile 2.00 % of companies score lower and have higher credit risk Established 1955 Experian Commercial IntelliscoreSM is an all-industry commercial model using business information to predict business risk. Its Sales (in millions) $65,030.00 predictiveness is among the best on the market today The objective of the Commercial Intelliscore Model is to predict seriously Employees 117,900 derogatory payment behavior. Possible score range from 0 to 100, where 0 is high risk and 100 is low risk Total OSHA Violations 18 -Liability Amount is the total dollar amount of debtor’s legal liability, OSHA is an arm of the Department of Labor that conducts inspections of company including accounts in collection, tax liens,judgments and/or bankruptcies facilities with the goal of preventing work-related injuries, illnesses and deaths. -The Number of Legal Derogatory items are the sum of Tax-Lien Worksites that do not meet health and/or safety standards at the time of inspection may Count, Bankruptcy,Judgment, Collection-Counter and UCC Derog receive an OSHA violation. Total FDA NDC Drugs 177 The total number of FDA Drugs filed in the FDA NDC Drug Database. Business Description Johnson & Johnson is engaged in the research and development, manufacture and sale of a range of products in the healthcare field. -

References Used in Algorithms for the Treatment of Persons with Crohn’S Disease
REFERENCES USED IN ALGORITHMS FOR THE TREATMENT OF PERSONS WITH CROHN’S DISEASE 1. AA Pharma Inc: Winpred (prednisone). In: CA Product Monograph. Vaughan, ON; 2018. 2. AbbVie Corporation: Humira (adalimumab). In: CA Product Monograph. St Laurent, QC; 2019. 3. AbbVie Inc: Humira (adalimumab). In: US Product Monograph. North Chicago, IL; 2020. 4. Amgen Canada Inc: Avsola (infliximab). In: CA Product Monograph. Mississauga, ON; 2020. 5. Amgen Inc: Amjevita (adalimumab-atto). In: US Product Monograph. Thousand Oaks, CA; 2019. 6. Amgen Inc: Avsola (infliximab-axxq). In: US Product Monograph. Thousand Oaks, CA; 2019. 7. Antares Pharma Inc: Methotrexate. In: FDA Product Monograph. Ewing, NJ; 2019. 8. Apotex Inc: Methotrexate. In: CA Product Monograph. Toronto, ON; 2019. 9. Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, Kito K, Sugimoto M, Andoh A: NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. Journal of gastroenterology 2016, 51(1):22-29. 10. Aspen Pharmacare Canada Inc: Imuran (azathioprine). In: CA Product Monograph. Oakville, ON; 2019. 11. Biogen Canada Inc: Tysabri (natalizumab). In: CA Product Monograph. Mississauga, ON; 2017. 12. Biogen Idec Inc: Tysabri (natalizumab). In: US Product Monograph. Cambridge, MA; 2019. 13. Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ et al: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clinical pharmacology and therapeutics 2015, 98(1):19-24. 14. Boehringer Ingelheim Pharmaceuticals Inc: Cyltezo (adalimumab-adbm). In: US Product Monograph. Ridgefield, CT; 2019. -
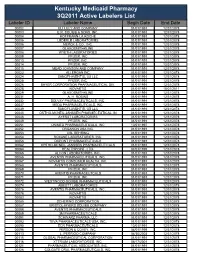
Active Labelers List Labeler ID Labeler Name Begin Date End Date 00002 ELI LILLY and COMPANY 01/01/1991 12/31/2078 00003 E.R
Kentucky Medicaid Pharmacy 3Q2011 Active Labelers List Labeler ID Labeler Name Begin Date End Date 00002 ELI LILLY AND COMPANY 01/01/1991 12/31/2078 00003 E.R. SQUIBB & SONS, INC. 01/01/1991 12/31/2078 00004 HOFFMANN-LA ROCHE 01/01/1991 12/31/2078 00005 LEDERLE LABORATORIES 01/01/1991 12/31/2078 00006 MERCK & CO., INC. 01/01/1991 12/31/2078 00007 GLAXOSMITHKLINE 01/01/1991 12/31/2078 00008 WYETH LABORATORIES 01/01/1991 12/31/2078 00009 PFIZER, INC 01/01/1991 12/31/2078 00013 PFIZER, INC. 01/01/1991 12/31/2078 00014 PFIZER, INC 01/01/1991 12/31/2078 00015 MEAD JOHNSON AND COMPANY 01/01/1991 12/31/2078 00023 ALLERGAN INC 01/01/1991 12/31/2078 00024 SANOFI-AVENTIS, US LLC 01/01/1991 12/31/2078 00025 PFIZER, INC. 01/01/1991 12/31/2078 00026 BAYER CORPORATION PHARMACEUTICAL DIV. 01/01/1991 12/31/2078 00028 NOVARTIS 01/01/1991 10/01/2011 00029 GLAXOSMITHKLINE 01/01/1991 12/31/2078 00031 A. H. ROBINS 01/01/1991 12/31/2078 00032 SOLVAY PHARMACEUTICALS, INC. 01/01/1991 12/31/2078 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 12/31/2078 00039 SANOFI-AVENTIS, US LLC 01/01/1991 12/31/2078 00045 ORTHO-MCNEIL-JANSSEN PHARMECEUTICAL, IN 01/01/1991 12/31/2078 00046 AYERST LABORATORIES 01/01/1991 12/31/2078 00049 PFIZER, INC 01/01/1991 12/31/2078 00051 UNIMED PHARMACEUTICALS, INC 10/01/1997 12/31/2078 00052 ORGANON USA INC. -

PFIZER INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2019 ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 1-3619 PFIZER INC. (Exact name of registrant as specified in its charter) Delaware 13-5315170 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification Number) 235 East 42nd Street, New York, New York 10017 (Address of principal executive offices) (zip code) (212) 733-2323 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common Stock, $.05 par value PFE New York Stock Exchange 0.000% Notes due 2020 PFE20A New York Stock Exchange 0.250% Notes due 2022 PFE22 New York Stock Exchange 1.000% Notes due 2027 PFE27 New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
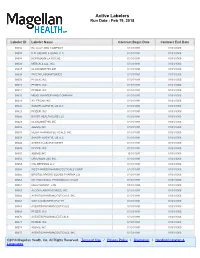
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD.