Pfizer's Bourla
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Health Industry Business Communications Council
Health Industry Business Communications Council Registered Labelers: Accredited Auto-ID Labeling Standards Argentina New MedTek Devices Pty Ltd Oxavita SRL Norseld Pty Ltd. Novadien Healthcare Pty Ltd The following companies Odontit S.A. (and/or their subsidiaries/ PAMPAMED S.R.L. Numedico Technologies Pty Ltd divisions) have applied PATEJIM SRL Opto Global Pty. Ltd. for a Labeler Identification Orthocell Limited Code (LIC) assignment with Austria Prolotus Technologies Pty Ltd HIBCC*. By doing so, they afreeze GmbH Red Milawa Pty Ltd dba Magic Mobility have demonstrated their AMI GmbH SDI Limited commitment to patient safety Bender Medsystems GmbH Signostics Ltd. and logistical efficiency for BHS Technologies GmbH Sirtex Medical Pty Ltd their customers, the industry Metasys Medizintechnik GmbH Smith & Nephew Surgical Pty. Ltd. and the public at large. PAA Laboratories GmbH Staminalift International Limited Safersonic Medizinprodukte Handels The Pipette Company Pty. Ltd. Any organization that is GmbH Thermo Electron Corporation interested in using the HIBC W & H Dentalwerk Burmoos GmbH Vush Pty Ltd uniform labeling system may apply for the assignment of VUSH STIMULATION Australia one or more LICs. William A Cook Australia Pty. Ltd. Adv. Surgical Design & Manufacture, Ltd. Last updated 9-21-2021 AirPhysio Pty Ltd Belgium Annalise-AI Pty Ltd 3M Europe Apollo Medical Imaging Technology Pty Advanced Medical Diagnostics SA/NV Ltd Analis SA/NV Benra Pty Ltd dba Gelflex Laboratories Baxter World Trade Bioclone Australia Pty. Ltd. Bio-Rad RSL Candelis, Inc. Bio-Rad Lab Inc Clinical Diag. Group DePuy Australia Pty. Ltd. Biosource Europe SA For more information, please dorsaVi Ltd Cilag NV contact the HIBCC office at: EC Certification Service GmbH Coris Bioconcept Fink Engineering Pty Ltd Fuji Hunt Photographic Chemicals NV 2525 E. -

How Jews Change the World - the Jerusalem Post
1/7/2021 How Jews change the world - The Jerusalem Post Jerusalem Post > Opinion How Jews change the world It comes as no surprise that two Jews, Bourla and Zaks, are spearheading two COVID- 19 vaccines to which many may one day soon owe their lives. By SHLOMO MAITAL DECEMBER 22, 2020 12:28 8000 British author Norman Lebrecht faces the media holding his Whitbread Book of the Year Award entry, 'The Song of Names,' in London in 2003. (photo credit: REUTERS) Advertisement ~ Listen to this article now \l:V 09:18 Powered by Trinity Audio https://www.jpost.com/jerusalem-report/how-jews-change-the-world-652199 1/6 1/7/2021 How Jews change the world - The Jerusalem Post Report~ A Greek Jew, two Turkish-born Germans, a Lebanese Armenian and an Israeli walk into a bar. Actually - not a bar, but a research lab. And it's not a joke. It's about brilliant people, all of them emigres, some Jewish, whose vaccines will hopefully save countless lives in Israel and the world. And there is a back story - about a century of Jewish genius that changed the world. It is recounted brilliantly by Norman Lebrecht, a British journalist and novelist, and graduate of Bar-llan University, in his book Genius and Anxiety: HowJews Changed the World, 1847-1947. Let's begin with the vaccine. On Friday, November 13, Prime Minister Benjamin Netanyahu signed a deal with the giant US pharmaceutical company Pfizer to purchase millions of coronavirus vaccine doses. It was only days after Pfizer announced clinical trials showed the vaccine was 90% effective at preventing COVID-19. -

Pfizer and Biontech Announce Collaboration with Biovac to Manufacture and Distribute COVID-19 Vaccine Doses Within Africa
Pfizer and BioNTech Announce Collaboration with Biovac to Manufacture and Distribute COVID-19 Vaccine Doses within Africa July 21, 2021 NEW YORK and MAINZ, GERMANY, July 21, 2021 — Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced the signing of a letter of intent with The Biovac Institute (Pty) Ltd, known as “Biovac”, a Cape Town-based, South African biopharmaceutical company, to manufacture the Pfizer-BioNTech COVID-19 vaccine for distribution within the African Union. Biovac will perform manufacturing and distribution activities within Pfizer’s and BioNTech’s global COVID-19 vaccine supply chain and manufacturing network, which will now span three continents and include more than 20 manufacturing facilities. To facilitate Biovac’s involvement in the process, technical transfer, on-site development and equipment installation activities will begin immediately. Pfizer and BioNTech expect that Biovac’s Cape Town facility will be incorporated into the vaccine supply chain by the end of 2021. Biovac will obtain drug substance from facilities in Europe, and manufacturing of finished doses will commence in 2022. At full operational capacity, the annual production will exceed 100 million finished doses annually. All doses will exclusively be distributed within the 55 member states that make up the African Union. “From day one, our goal has been to provide fair and equitable access of the Pfizer-BioNTech COVID-19 vaccine to everyone, everywhere,” said Albert Bourla, Chairman and Chief Executive Officer, Pfizer. “Our latest collaboration with Biovac is a shining example of the tireless work being done, in this instance to benefit Africa. We will continue to explore and pursue opportunities to bring new partners into our supply chain network, including in Latin America, to further accelerate access of COVID-19 vaccines.” “We aim to enable people on all continents to manufacture and distribute our vaccine while ensuring the quality of the manufacturing process and the doses,” said Ugur Sahin, M.D., CEO and Co-founder of BioNTech. -

Proxy Statement 2021
Purpose Blueprint OUR PURPOSE Breakthroughs that change patients’ lives OUR BOLD MOVES 1. Unleash the 2. Deliver 3. Transform our 4. Win the 5. Lead the power of our first-in-class go-to-market digital race conversation people science model in pharma OUR BIG IDEAS 1.1 Create room for 2.1 Source the best 3.1 Improve access 4.1 Digitize drug 5.1 Be known as the meaningful work science in the through new payer discovery and most patient- 1.2 Recognize both world partnerships development centric company leadership and 2.2 Double our 3.2 Address the 4.2 Enhance health 5.2 Drive pro- performance innovation success patient outcomes and innovation/pro- 1.3 Make Pfizer an rate affordability patient experience patient policies amazing 2.3 Bring medicines to challenge 4.3 Make our work 5.3 Focus the narrative workplace for all the world faster 3.3 Transform the way faster and easier on the value of our we engage science patients and physicians OUR VALUES Courage Excellence Equity Joy A Letter from Pfizer’s Chairman & Chief Executive Officer To Our Shareholders: 2020 was a year like none other in Pfizer’s history – defined by bold decisions, even bolder actions and incredible results. With the separation of Upjohn, we created a company that was 20% smaller, but more focused than ever on delivering first-in-class science for the benefit of patients. Through our collaboration with BioNTech, we delivered a breakthrough COVID-19 vaccine in less than a year. And by harnessing the power of a variety of digital capabilities – as well as our own steadfast commitment to patients – we made sure that despite lockdowns and travel restrictions, we continued to reach more than 400 million patients worldwide with our medicines and vaccines. -

Pfizer and Biontech Receive First U.S. Authorization for Emergency Use of COVID-19 Vaccine in Adolescents
Pfizer and BioNTech Receive First U.S. Authorization for Emergency Use of COVID-19 Vaccine in Adolescents May 11, 2021 In a Phase 3 trial, the vaccine was 100% effective and generally well tolerated in participants aged 12 to 15 years Data also submitted to European Medicines Agency (EMA) and other global regulators, with additional authorizations expected in coming weeks NEW YORK and MAINZ, GERMANY, May 11, 2021 —Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) announced today that the U.S. Food and Drug Administration (FDA) has expanded the Emergency Use Authorization (EUA) for their COVID-19 vaccine to include individuals 12 to 15 years of age. This is the first COVID-19 vaccine authorized in the U.S. for use in this age group. “Today’s expansion of our EUA represents a significant step forward in helping the U.S. government broaden its vaccination program and help protect adolescents before the start of the next school year,” said Albert Bourla, Chairman and Chief Executive Officer, Pfizer. “We are grateful to all of our clinical trial volunteers and their families, whose courage helped make this milestone possible. Together, we hope to help bring a sense of normalcy back to young people across the country and around the world.” The FDA based its decision on data from a pivotal Phase 3 clinical trial, which enrolled 2,260 participants aged 12 to 15 years. Topline results from this trial, announced on March 31, 2021, showed a vaccine efficacy of 100% in participants with or without prior SARS-CoV-2 infection and robust antibody responses. -

April 16, 2021
April 16, 2021 Dear <<First Name>>, Welcome to this week's issue of the California Pharmacists Association's CEO Message. IN THIS ISSUE Emerging Role of Pharmacists on the Front Line of Vaccine Administration Now that we have been at the front and center of this whole vaccine for COVID-19 and its protocols, the role of pharmacists as a frontline vaccinator and administration has really emerged with this pandemic. [Read Article] Burnout and the Challenges Facing Pharmacists during COVID-19: Results of a National Survey Background COVID-19 has impacted the psychological wellbeing of healthcare workers and has forced pharmacists to adapt their services. [Read Article] Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine As of April 12, more than 6.8 million doses of the Johnson & Johnson (Janssen) vaccine have been administered in the U.S. CDC and FDA are reviewing data involving six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving the J&J vaccine. [Read Article] How Pharmacists Can Protect Patients from Outdated COVID-19 Treatment Hydroxychloroquine is an ineffective and potentially dangerous treatment for COVID-19, yet prescriptions for patients with the virus continue. [Read Article] Pfizer CEO says Third Shot Likely Needed Within 12 Months People will probably need a third shot of the Pfizer/BioNTech within 12 months of being fully vaccinated, Pfizer CEO Albert Bourla told CNBC on Thursday. Annual shots may also be needed, Bourla said. [Read Article] Tabula Rasa HealthCare Expands COVID-19 Vaccine Support Services to Community Pharmacies Tabula Rasa HealthCare, Inc., a leading healthcare technology company advancing the safe use of medication, today announced an expansion of COVID-19 services for independent and chain pharmacies. -

Just a Nosh..Just
St. Petersburg, FL 33707 St. Petersburg, FL Avenue 6416 Central Tampa Jewish Press of Inc. Bay, Tampa The Jewish Press Group of www.jewishpresstampa.com VOL.33, NO. 9 TAMPA, FLORIDA A NOVEMBER 20 - DECEMBER 3, 2020 12 PAGES Pompeo visits settlements; sets policy on BDS, ‘made in Israel’ (JTA) – Mike Pompeo became the Pompeo also said that the Trump ad- first U.S. secretary of state to visit both ministration will cut ties with any The Jewish Press Group an Israeli settlement in the West Bank groups that support the boycott Is- PAID U.S. POSTAGE of Tampa Bay, Inc. Bay, Tampa of PRESORTED and the Golan Heights, a disputed area rael movement, (commonly known STANDARD on Israel’s border with Syria that the as Boycott, Divestment, Sanctions Trump administration has recognized movement or BDS) which the U.S. as part of Israel. will officially consider anti-Semitic. Twitter Pompeo Mike Photo At a news conference Thursday, Later in the day, he visited the Secretary of State Mike Pompeo leads a delegation to the Nov. 19, in Jerusalem with Israeli Psagot winery in a settlement near West Bank town of Qasr al-Yahud, the site where tradition Prime Minister Benjamin Netanyahu, POMPEO continued on PAGE 11 holds that Jesus was baptized. VACCINE HEROES JustJust aaJTA newsnosh..nosh.. service In-person Hanukkah party planned at White House Israeli scientist Immigrant helps make WASHINGTON – The White House is throwing an in- person Hanukkah party, one of a series of recent events at Moderna history with Pfizer’s vaccine the Trump administration has held despite coronavirus concerns. -

Report from Accountable.US
NEW ANALYSIS REVEALS $250M IN INSIDER PROFITEERING AT GOVERNMENT-BACKED DRUG COMPANIES, INCLUDING $105M IN NEW TRADES IN LESS THAN 3 MONTHS’ TIME In Six Months Since Launch Of Operation Warp Speed, Execs At Five Drug Companies Made Net Profit Of More Than $250 Million Dumping Their Companies’ Stocks. An earlier report from Accountable.US compiled executive trading data from the launch of Operation Warp Speed on May 15 through August 31, 2020 and found they made stock transactions valued at a net profit of more than $145 million. New analysis of insider transactions at those companies over the six months between May 15 and November 15, 2020, show more than $250 million in net profit. Company Net Profit Emergent BioSolutions $23,906,515.14 Johnson & Johnson $4,276,630.00 Moderna $165,889,439.69 Novavax $46,372,082.90 Pfizer $10,490,165.85 Total $250,934,833.57 From September To Mid-November, Execs At Four Drug Companies Involved In Operation Warp Speed Have Made Net Profit Of More Than $105M Dumping Their Companies’ Stock. According to SEC filings, from the beginning of September through November 15, 2020, executives and directors at four of the companies receiving COVID vaccine funding through Operation Warp Speed have made stock transactions valued at a net profit of more than $105 million. NOTE: Johnson & Johnson did not report any new individual insider transactions during this period. Company Net Profit Emergent BioSolutions $18,860,527.37 Moderna $50,406,146.77 Novavax $29,037,668.05 Pfizer $7,388,569.80 Total $105,692,911.99 • The net profit of these transactions from May 15-November 15, 2020 was $250.9 million; the total profit of these trades was $292.1 million. -

April 28, 2021
American Nephrology Nurses Association Daily Capitol Hill Update – Wednesday, April 28, 2021 The following information comes from directly from news sources including Bloomberg Government, Kaiser Health News, and other news sources. Schedules: White House and Congress WHITE HOUSE • 9:00 PM: President Biden addresses a joint session of Congress. CONGRESS: • The Senate is set to take up a measure today that would reinstate an Obama-era rule limiting harmful methane emissions from oil and gas drilling. • The Senate Judiciary Committee’s initial hearing for Biden’s first judicial nominations, including D.C. Circuit nominee Ketanji Brown Jackson, will take place today. Congressional, Health Policy, and Political News • Bloomberg Government: Medicare Age Eligibility: Biden’s new American Families Plan calls for the lowering of the age to 60 for people to voluntarily enroll in Medicare, according to a White House fact sheet. Congressional Democrats have supported such a move, and want to help fund the benefits expansion with $456 billion that Medicare could save over 10 years through Biden’s plan to let the program negotiate its own drug prices. o After the massive loss of jobs and employer-based health coverage due to Covid- 19, Democrats say public support for their plan is strong. A recent Gallup poll found roughly 46 million Americans couldn’t pay for quality health care if they needed it. And even before the pandemic struck, 77% of Americans, including 69% of Republicans, favored letting adults ages 50 to 64 buy into the Medicare program, according to a January 2019 poll by the Kaiser Family Foundation. -

Jewish Reporter a Publication of the Flint Jewish Federation
Jewish Reporter A Publication of the Flint Jewish Federation VOLUME. 72 NO. 4 APRIL 1, 2021, 19 NISAN 5781 VOLUME. 72 NO. 4 VVAT, 5777 APRIL 1, 2021, 19 NISAN 5781 IS PUBLISHED WILL YOU LEAVE A LEGACY? MONTHLY BY THE Flint Jewish Federation Every five years. You should review your will and estate plan every five years to 5080 West Bristol Rd. Ste.3 make sure it still expresses your wishes. Has it been a while? Or have you recently Flint, MI 48507 updated your estate plan? Did you leave a legacy to the Flint Jewish community— Office: 810-767-5922 Fax: 810-767-9024 TBE, CBI, the Federation, Machpelah Cemetery or Chabad? Email: [email protected] If you did let us know by completing and submitting a Letter of Intent. You don’t Website: flintfed.com need to share the amount if you don’t want to but let us know if you made a gift by will, life insurance policy, pension fund, or some other means and to whom. Any of our Steven C. Low Jewish Institutions that has 18 Letters of Intent submitted will earn a $5000 gift from Executive Director the Harold Grinspoon Foundation. Sheryl Deutsch You don’t have to pay anything right now—just fill out the form, submit it and within President the year formalize the gift. If you already have formalized the gift then submitting Jeff Himelhoch the Letter is all you need to do for your gift to count. Vice President Federation has already received three Letters. Three families so far have made a commitment to create a legacy to ensure a future flourishing Flint Jewish Community. -
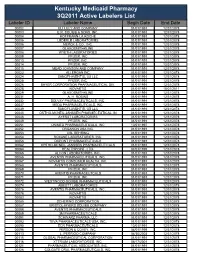
Active Labelers List Labeler ID Labeler Name Begin Date End Date 00002 ELI LILLY and COMPANY 01/01/1991 12/31/2078 00003 E.R
Kentucky Medicaid Pharmacy 3Q2011 Active Labelers List Labeler ID Labeler Name Begin Date End Date 00002 ELI LILLY AND COMPANY 01/01/1991 12/31/2078 00003 E.R. SQUIBB & SONS, INC. 01/01/1991 12/31/2078 00004 HOFFMANN-LA ROCHE 01/01/1991 12/31/2078 00005 LEDERLE LABORATORIES 01/01/1991 12/31/2078 00006 MERCK & CO., INC. 01/01/1991 12/31/2078 00007 GLAXOSMITHKLINE 01/01/1991 12/31/2078 00008 WYETH LABORATORIES 01/01/1991 12/31/2078 00009 PFIZER, INC 01/01/1991 12/31/2078 00013 PFIZER, INC. 01/01/1991 12/31/2078 00014 PFIZER, INC 01/01/1991 12/31/2078 00015 MEAD JOHNSON AND COMPANY 01/01/1991 12/31/2078 00023 ALLERGAN INC 01/01/1991 12/31/2078 00024 SANOFI-AVENTIS, US LLC 01/01/1991 12/31/2078 00025 PFIZER, INC. 01/01/1991 12/31/2078 00026 BAYER CORPORATION PHARMACEUTICAL DIV. 01/01/1991 12/31/2078 00028 NOVARTIS 01/01/1991 10/01/2011 00029 GLAXOSMITHKLINE 01/01/1991 12/31/2078 00031 A. H. ROBINS 01/01/1991 12/31/2078 00032 SOLVAY PHARMACEUTICALS, INC. 01/01/1991 12/31/2078 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 12/31/2078 00039 SANOFI-AVENTIS, US LLC 01/01/1991 12/31/2078 00045 ORTHO-MCNEIL-JANSSEN PHARMECEUTICAL, IN 01/01/1991 12/31/2078 00046 AYERST LABORATORIES 01/01/1991 12/31/2078 00049 PFIZER, INC 01/01/1991 12/31/2078 00051 UNIMED PHARMACEUTICALS, INC 10/01/1997 12/31/2078 00052 ORGANON USA INC. -

PFIZER INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2019 ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 1-3619 PFIZER INC. (Exact name of registrant as specified in its charter) Delaware 13-5315170 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification Number) 235 East 42nd Street, New York, New York 10017 (Address of principal executive offices) (zip code) (212) 733-2323 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common Stock, $.05 par value PFE New York Stock Exchange 0.000% Notes due 2020 PFE20A New York Stock Exchange 0.250% Notes due 2022 PFE22 New York Stock Exchange 1.000% Notes due 2027 PFE27 New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.