2019 Annual Meeting Education Committee All Relationships Are Considered Compensated
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moderna Appoints Oncology Leader Dr. Stephen Kelsey As President of Onkaido
Moderna Appoints Oncology Leader Dr. Stephen Kelsey as President of Onkaido Former Genentech executive to lead Moderna’s first venture company, focused exclusively in novel biology for oncology drug development CAMBRIDGE, Mass., July 1, 2014— Moderna Therapeutics, the pioneer in developing messenger RNA (mRNA) TherapeuticsTM, a revolutionary treatment modality to enable the in vivo production of therapeutic proteins, announced today that Stephen Kelsey, M.D., will become president of Onkaido Therapeutics, Moderna’s oncology drug development company, effective July 21. Launched in January of this year, Onkaido is Moderna’s first venture company, focused exclusively on developing and commercializing mRNA-based oncology treatments. “As we continue to grow Moderna and perfect our mRNA Therapeutics platform, we are also focused on building a transformational oncology company that will benefit patients and society. This requires hiring the best oncology talent to lead Onkaido,” said Stéphane Bancel, president and founding chief executive officer, Moderna. “Steve brings a wealth of experience in oncology drug development to his new role. His knowledge and leadership, combined with the team of Onkaido scientists and Moderna’s innovative mRNA technology, will help speed a new class of cancer drugs to patients around the world.” Dr. Kelsey has extensive pharmaceutical industry experience in oncology. After 16 years as an academic clinician, he started his industry career at Sugen, and later was vice president of hematology/oncology at Genentech. While at Genentech, Dr. Kelsey played a significant role in the development of key products Perjeta®, Kadcyla® and Erivedge®, as well as other molecules in the company’s oncology portfolio. He left Genentech in 2009 to run Geron’s oncology division, where he served for four years as executive vice president, research and development, and chief medical officer helping to develop therapeutics and vaccines to fight cancer. -

Amgen and Incyte – the Biotechnology Acquisition
Amgen and Incyte – the biotechnology acquisition Ana Carolina Coelho Student number: 152416013 Dissertation written under the supervision of António Luís Borges de Assunção Dissertation submitted in partial fulfilment of requirements for the MSc in Finance, at the Universidade Católica Portuguesa, May 2018 Abstract The main goal of this dissertation is to study the hypothesis of an acquisition in the biotechnology industry, between Amgen (acquirer) and Incyte (target). Amgen is a U.S.-based biotechnology company currently facing a decrease in sales, mainly due to an increase in the market share of generic drugs and the rise of biosimilar products. To offset the poor performance, it is looking for an acquisition in its industry, for which Incyte could be a suitable target. Incyte belongs to the U.S.-biotechnology industry, and although it has a shy presence in the market with only two released drugs, its revenues are expected to increase significantly in the near future. The combination of these companies would allow knowledge transfer about research and development process of new drugs, a stronger position in Europe, and a higher investment power to apply in R&D. The combined company would be able to decrease the number of employees due to duplication of jobs and decrease the cost of sales, as the power over suppliers increases. The potential synergies are valued at $28,522.8 million, of which $16,739.2 million are more likely to be realized. The combined firm, after the introduction of synergies, is expected to generate the same or even higher returns than the sum of the stand- alone businesses and higher growth rates, fulfilling Amgen’s need of growth. -

Kopi Af Aktivlisten 2021-06-30 Ny.Xlsm
Velliv noterede aktier i alt pr. 30-06-2021 ISIN Udstedelsesland Navn Markedsværdi (i DKK) US0378331005 US APPLE INC 1.677.392.695 US5949181045 US MICROSOFT CORP 1.463.792.732 US0231351067 US AMAZON.COM INC 1.383.643.996 DK0060534915 DK NOVO NORDISK A/S-B 1.195.448.146 US30303M1027 US FACEBOOK INC-CLASS A 1.169.094.867 US02079K3059 US ALPHABET INC-CL A 867.740.769 DK0010274414 DK DANSKE BANK A/S 761.684.457 DK0060079531 DK DSV PANALPINA A/S 629.313.827 US02079K1079 US ALPHABET INC-CL C 589.305.120 US90138F1021 US TWILIO INC - A 514.807.852 US57636Q1040 US MASTERCARD INC - A 490.766.560 US4781601046 US JOHNSON & JOHNSON 478.682.981 US70450Y1038 US PAYPAL HOLDINGS INC 471.592.728 DK0061539921 DK VESTAS WIND SYSTEMS A/S 441.187.698 US79466L3024 US SALESFORCE.COM INC 439.114.061 US01609W1027 US ALIBABA GROUP HOLDING-SP ADR 432.325.255 US8835561023 US THERMO FISHER SCIENTIFIC INC 430.036.612 US22788C1053 US CROWDSTRIKE HOLDINGS INC - A 400.408.622 KYG875721634 HK TENCENT HOLDINGS LTD 397.054.685 KR7005930003 KR SAMSUNG ELECTRONICS CO LTD 389.413.700 DK0060094928 DK ORSTED A/S 378.578.374 ES0109067019 ES AMADEUS IT GROUP SA 375.824.429 US46625H1005 US JPMORGAN CHASE & CO 375.282.618 US67066G1040 US NVIDIA CORP 357.034.119 US17275R1023 US CISCO SYSTEMS INC 348.160.692 DK0010244508 DK AP MOLLER-MAERSK A/S-B 339.783.859 US20030N1019 US COMCAST CORP-CLASS A 337.806.502 NL0010273215 NL ASML HOLDING NV 334.040.559 CH0012032048 CH ROCHE HOLDING AG-GENUSSCHEIN 325.008.200 KYG970081173 HK WUXI BIOLOGICS CAYMAN INC 321.300.236 US4370761029 US HOME DEPOT INC 317.083.124 US58933Y1055 US MERCK & CO. -

Print Layout 1
TECHNICAL PROGRAM Monday, March 19 5:00 – 8:00 pm Welcome Reception Tuesday, March 20 8:00 – 10:00 Session 1: Setting the Conference Context and Drivers Chair: Geoff Slaff (Amgen) Roger Perlmutter (Amgen) Conquering the Innovation Deficit in Drug Discovery Helen Winkle (CDER, FDA) Regulatory Modernization 10:00 – 10:30 Break Vendor and poster review 10:30 – 12:30 Session 2: Rapid Cell Line Development and Improved Expression System Development Chairs: Timothy Charlebois (Wyeth) and John Joly (Genentech) Amy Shen (Genentech) Stable Antibody Production Cell-Line Development with an Improved Selection Process and Accelerated Timeline Mark Leonard (Wyeth) High-Performing Cell-Line Development within a Rapid and Integrated Platform Process Control Pranhitha Reddy (Amgen) Applying Quality-by-Design to Cell Line Development Lin Zhang (Pfizer) Development of a Fully-Integrated Automated System for High-Throughput Screening and Selection of Single Cells Expressing Monoclonal Antibodies 12:30 – 2:00 Lunch Vendor and poster review 2:00 – 4:30 Session 3: High-Throughput Bulk Process Development Chairs: Brian Kelley (Wyeth) and Jorg Thommes (Biogen Idec) Colette Ranucci (Merck) Development of a Multi-Well Plate System for High-Throughput Process Development Min Zhang (SAFC Biosciences) CHO Media Library – an Efficient Platform for Rapid Development and Optimization of Cell Culture Media Supporting High Production of Pharmaceutical Proteins in Chinese Hamster Ovary Cells Nigel Titchener-Hooker The Use of Ultra-Scale-Down Approaches to Enable Rapid Investigation -

Genentech Tocilizumab Letter of Authority June 24 2021
June 24, 2021 Hoffmann-La Roche, Ltd. C/O Genentech, Inc. Attention: Dhushy Thambipillai Regulatory Project Management 1 DNA Way, Bldg 45-1 South San Francisco, CA 94080 RE: Emergency Use Authorization 099 Dear Ms. Thambipillai: This letter is in response to Genentech, Inc.’s (Genentech) request that the Food and Drug Administration (FDA) issue an Emergency Use Authorization (EUA) for the emergency use of Actemra1 (tocilizumab) for the treatment of coronavirus disease 2019 (COVID-19) in certain hospitalized patients, as described in the Scope of Authorization (Section II) of this letter, pursuant to Section 564 of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. §360bbb-3). On February 4, 2020, pursuant to Section 564(b)(1)(C) of the Act, the Secretary of the Department of Health and Human Services (HHS) determined that there is a public health emergency that has a significant potential to affect national security or the health and security of United States citizens living abroad, and that involves the virus that causes coronavirus disease 2019 (COVID-19).2 On the basis of such determination, the Secretary of HHS on March 27, 2020, declared that circumstances exist justifying the authorization of emergency use of drugs and biological products during the COVID-19 pandemic, pursuant to Section 564 of the Act (21 3 U.S.C. 360bbb-3), subject to terms of any authorization issued under that section. Actemra is a recombinant humanized monoclonal antibody that selectively binds to both soluble and membrane-bound human IL-6 receptors (sIL-6R and mIL-6R) and subsequently inhibits IL- 6-mediated signaling through these receptors. -

Inozyme Pharma Expands Medical Leadership Team
Inozyme Pharma Expands Medical Leadership Team Strengthens Inozyme’s Ability to Advance Lead Candidate, INZ-701, into Clinical Trials Boston, Mass., Nov. 14, 2019 – Inozyme Pharma Inc., a biotechnology company developing novel medicines to treat rare and life-threatening mineralization disorders, today announced the addition of three industry veterans to its leadership team: • Pedro Huertas M.D., Ph.D., as Chief Medical Officer, • Gus Khursigara Ph.D., as Vice President of Medical Affairs and Clinical Operations, and • Catherine Nester, as Vice President of Physician and Patient Strategies. The executives will be instrumental in advancing Inozyme’s lead drug candidate, INZ-701, into clinical trials in 2020 for the treatment of patients with ENPP1 deficiency. INZ-701 is the first therapy that addresses the pathology of ENPP1 deficiency, including diseases such as generalized arterial calcification of infancy (GACI) type 1 and autosomal recessive hypophosphatemic rickets type 2 (ARHR2), both of which are rare and life-threatening manifestations of this enzyme deficiency. Inozyme Pharma received orphan drug designation for INZ-701 in the US and EU in 2018. “We are pleased and excited to welcome Pedro, Gus and Catherine to the Inozyme team,” said Axel Bolte, co-founder and chief executive officer of Inozyme. “Their expertise in orphan drug development – spanning medical affairs, regulatory affairs and clinical development – will strengthen and accelerate our ability to bring potentially life-saving medicines to people who urgently need effective treatments.” Dr. Huertas, a veteran of the pharmaceutical industry, has extensive experience in research and development and medical and regulatory affairs, especially regarding rare disorders and enzyme replacement therapies. -

EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
December 22, 2008 EP Vantage Interview - Silence hoping to have something to shout about in 2009 Lisa Urquhart With its first product expected to go into the clinic potentially as early as January, Silence Therapeutics is hoping that 2009 will be year it has something to shout about and one that will reverse the alarming share price decline that has seen the company’s valuation slip from a high of over £170m in June 2007 to £21.6m today. Silence is one of a growing number of companies working in RNA interference (RNAi) and particularly short interfering RNA (siRNA), which work by selectively silencing or inactivating genes related to certain diseases. It is importantly one of only two companies that have composition of matter patent protection for their siRNA drugs. Speaking to EP Vantage, Iain Ross, chief executive of Silence, says: “It’s a big year coming up for us.” The group has spent most of the last 12 months strengthening its IP position, and next year comes the important move of the group’s lead candidate Atu027 into the clinic, an event Mr Ross says should take place in the first quarter of the year. Many expect it could be as soon as the end of January. Partnering focus What is less expected is the speed at which Mr Ross intends to partner the drug, which is being developed in solid tumours, with an emphasis on lung cancer. “We would be looking at doing something either at the end of 2009 or the beginning of 2010,” he says. Key to partnering discussions will be the drug demonstrating good safety data in a number of cancer patients, which could be the catalyst to starting talks with big pharma who have already started to ask about Atu027. -
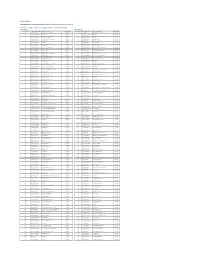
Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN)
Date: 20-Aug-21 Index Weights as of monthly rebalance date 10-Aug-21 Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN) Long Exposure Short Exposure Constituent Bloomberg Ticker Constituent Name Weight(%) Constituent Bloomberg Ticker Constituent Name Weight(%) 1 AAP UN Equity Advance Auto Parts Inc 0.24% 1 A UN Equity Agilent Technologies Inc -0.12% 2 ABBV UN Equity AbbVie Inc. 0.59% 2 HWM UN Equity Alcoa Inc -1.02% 3 ABC UN Equity AmerisourceBergen Corp 0.06% 3 AAL UW Equity American Airlines Group Inc -1.09% 4 ADBE UW Equity Adobe Systems Inc 0.01% 4 AAPL UW Equity Apple Inc. -0.46% 5 ADM UN Equity Archer-Daniels-Midland Co 0.26% 5 ABMD UW Equity ABIOMED Inc -0.11% 6 ADSK UW Equity Autodesk Inc 0.26% 6 ABT UN Equity Abbott Laboratories -0.26% 7 AES UN Equity AES Corp 0.37% 7 CB UN Equity ACE Limited -0.07% 8 AIG UN Equity American Intl Group Inc 0.52% 8 ACN UN Equity Accenture plc -0.29% 9 AIZ UN Equity Assurant Inc 0.11% 9 ADI UW Equity Analog Devices Inc -0.13% 10 ALGN UW Equity Align Technology Inc 0.59% 10 ADP UW Equity Automatic Data Processing -0.76% 11 ALL UN Equity Allstate Corp 0.16% 11 AEE UN Equity Ameren Corp -0.24% 12 ALLE UN Equity Allegion PLC 0.34% 12 AEP UW Equity American Electric Power -0.23% 13 AMAT UW Equity Applied Materials Inc 0.59% 13 AFL UN Equity AFLAC Inc -0.29% 14 AMD UW Equity Advanced Micro Devices Inc 1.15% 14 AJG UN Equity ARTHUR J GALLAGHER & CO -0.23% 15 AME UN Equity AMETEK Inc 0.26% 15 AKAM UW Equity Akamai Technologies Inc -0.11% 16 AMT UN Equity American Tower Corp A 0.39% 16 ALB UN -

IQVIA Pharma Deals Half-Year Review of 2020
White Paper IQVIA Pharma Deals Half-Year Review of 2020 HEATHER CARTWRIGHT, Senior Analyst, Global Market Insights, IQVIA MICHELLE LIU, Analyst, Global Market Insights, IQVIA TASKIN AHMED, Manager, Global Market Insights, IQVIA Table of contents Introduction 1 Uncertainty and prudence cause M&A to stall 2 Licensing deal values continue to rise 5 Roche pips AstraZeneca to title of most active dealmaker 10 COVID-19 pandemic drives increase in R&D collaboration 11 Infectious diseases replace oncology as top therapeutic area for dealmaking 14 Outlook for H2 2020 15 About the authors 17 Introduction COVID-19 shakes up the dealmaking landscape H1 2020 was an unprecedented time for dealmaking in the life sciences sector. While the COVID-19 pandemic rapidly provoked a significant level of partnering activity amongst biopharma companies keen to expedite the development of vaccines or therapeutics to tackle the virus, it also acted as a brake on other types of dealmaking, most notably M&A which saw a sharp decline in activity as a result of uncertainty and operational disruption. Indeed, H1 2020 was notable for the absence of any US$5 B deals on the scale seen in previous years as companies instead focused their attentions on internal programs and potential COVID-19 solutions, opting only for a few asset-driven acquisitions. There was also plentiful capital available to allow biotech companies to stay independent, particularly those in the US. As a result, aggregate spending on M&A by life science companies plummeted to just US$19.1 B in the first 6 months of 2020. -

Incyte Corporation 2016 Annual Report
2016 ANNUAL REPORT TABLE OF CONTENTS Letter to Shareholders 2 Key Planned Goals for 2017 6 Innovation 7 Growth 10 Strength 13 Corporate Responsibility 15 Company Information 19 ii Incyte’s Executive Management Team LETTER TO SHAREHOLDERS Standing, left to right: Steven H. Stein, MD; Dear Shareholders, Paula J. Swain; Wenqing Yao, PhD; Jonathan E. Dickinson; At Incyte, we believe that innovation and the discovery of new products David W. Gryska; creates long-term value for patients and society, as well as for our employ- Hervé Hoppenot; Michael Morrissey; ees and our shareholders. It is our commitment to these objectives that has Vijay Iyengar, MD enabled us to make significant progress in the last year. During 2016, we saw continued growth in the number of patients being treated with Jakafi® Seated, left to right: Eric H. Siegel, JD, MBA; (ruxolitinib), our JAK1/JAK2 inhibitor, and we also added Iclusig® (ponati- Reid M. Huber, PhD; nib) to our commercial portfolio as part of our European transaction with Barry P. Flannelly, PharmD, MBA ARIAD Pharmaceuticals, Inc. In February 2017, with Eli Lilly & Company, we announced the European approval of Olumiant® (baricitinib). I believe that we are on Summary of Revenue-Generating Products track to reach our goal of PRODUCT NAME DRUG NAME TARGET APPROVED TERRITORY INDICATION(S) becoming a world-class, Jakafi® ruxolitinib1 JAK1/JAK2 Global MF3; PV4 Jakavi® global biopharmaceutical organization. For the first Iclusig® ponatinib BCR-ABL Europe CML and Ph+ ALL5 time in the history of our company, Incyte’s total Olumiant® baricitinib2 JAK1/JAK2 Europe RA6 yearly revenue surpassed $1 billion in 2016. -

Massmutual Mid Cap Growth Fund T
Fund Holdings As of 06/30/2021 MassMutual Mid Cap Growth Fund T. Rowe Price | Frontier Capital Prior to 5/1/2021, the Fund name was MassMutual Select Mid Cap Growth Fund. Fund Shares or Par Position Market Security Name Ticker CUSIP Weighting (%) Amount Value ($) Microchip Technology Inc MCHP 595017104 2.07 1,348,381 201,906,571 Agilent Technologies Inc A 00846U101 1.90 1,252,717 185,164,100 Hologic Inc HOLX 436440101 1.84 2,678,942 178,739,010 Teleflex Inc TFX 879369106 1.82 441,297 177,308,722 Ball Corp BLL 058498106 1.75 2,104,895 170,538,593 Textron Inc TXT 883203101 1.55 2,199,000 151,225,230 Burlington Stores Inc BURL 122017106 1.54 465,929 150,024,479 Catalent Inc CTLT 148806102 1.53 1,376,000 148,773,120 Marvell Technology Inc MRVL 573874104 1.46 2,442,368 142,463,325 Bruker Corp BRKR 116794108 1.39 1,776,000 134,940,480 Ingersoll Rand Inc IR 45687V106 1.29 2,564,000 125,148,840 The Cooper Companies Inc COO 216648402 1.28 314,645 124,684,374 KKR & Co Inc Ordinary Shares KKR 48251W104 1.25 2,050,813 121,490,162 Caesars Entertainment Inc CZR 12769G100 1.13 1,057,315 109,696,431 Reserve Invt Fds 0 76105Y208 1.07 103,877,547 103,877,547 DocuSign Inc DOCU 256163106 1.05 366,000 102,322,620 Chipotle Mexican Grill Inc CMG 169656105 1.04 65,283 101,210,846 Dollar General Corp DG 256677105 1.02 460,009 99,541,348 Veeva Systems Inc Class A VEEV 922475108 1.00 314,189 97,697,070 Avantor Inc AVTR 05352A100 0.98 2,700,000 95,877,000 JB Hunt Transport Services Inc JBHT 445658107 0.98 585,000 95,325,750 KLA Corp KLAC 482480100 0.97 291,710 94,575,299 -

CDER – Redi: Focus on CGMP & FDA Inspections – Participant List
FDA – CDER – RedI: Focus on CGMP & FDA Inspections – Participant List These participants granted permission to share their contact information Eileen Zhou Michael Channing A B M Mahfuz ul Alam Research Associate Group Chief - Positron Emission General Manager, Quality Operations Neuralstem Tomography Department ACI HealthCare Limited Germantown, MD, United States NIH Dhaka, Non-US, Bangladesh [email protected] Bethesda, MD, United States [email protected] [email protected] Abe Wong ABHIJEET GUJAR Adam Ebbinghouse CCO Chief Operating Officer QC Associate Gmpsigma SETHU KP Pharmaceutical Technology, Inc. Seattle, WA, United States Porvorim Goa, Non-US, India Bloomington, IN, United States [email protected] [email protected] [email protected] Adil Gatrad Adiseshu Modugula Aditiben Patel Director, Quality Systems RA Regulatory Specialist Actavis MSN USAMMDA Parsippany, NJ, United States Hyderabad, Non-US, India Frederick, MD, United States [email protected] [email protected] [email protected] Adriana de la Cruz ahsanul haque Aimee Gogarty Manager CSO Quality Coordinator Laboratorios Pisa SA de CV FDA Mallinckrodt Jalisco, Non-US, Mexico silver spring, MD, United States Port Allen, LA, United States [email protected] [email protected] [email protected] Aislyn Fronzak Ajay Deshmukh Ajay Khedkar QC Manager QA manager Regulatory Affair PL Developments Ingenus Pharmaceutical Umedica Laboratories Pvt Ltd Clinton, SC, United States Navi mumbai, Non-US, India Mumbai, Non-US, India [email protected]