Incyte Corporation 2016 Annual Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amgen and Incyte – the Biotechnology Acquisition
Amgen and Incyte – the biotechnology acquisition Ana Carolina Coelho Student number: 152416013 Dissertation written under the supervision of António Luís Borges de Assunção Dissertation submitted in partial fulfilment of requirements for the MSc in Finance, at the Universidade Católica Portuguesa, May 2018 Abstract The main goal of this dissertation is to study the hypothesis of an acquisition in the biotechnology industry, between Amgen (acquirer) and Incyte (target). Amgen is a U.S.-based biotechnology company currently facing a decrease in sales, mainly due to an increase in the market share of generic drugs and the rise of biosimilar products. To offset the poor performance, it is looking for an acquisition in its industry, for which Incyte could be a suitable target. Incyte belongs to the U.S.-biotechnology industry, and although it has a shy presence in the market with only two released drugs, its revenues are expected to increase significantly in the near future. The combination of these companies would allow knowledge transfer about research and development process of new drugs, a stronger position in Europe, and a higher investment power to apply in R&D. The combined company would be able to decrease the number of employees due to duplication of jobs and decrease the cost of sales, as the power over suppliers increases. The potential synergies are valued at $28,522.8 million, of which $16,739.2 million are more likely to be realized. The combined firm, after the introduction of synergies, is expected to generate the same or even higher returns than the sum of the stand- alone businesses and higher growth rates, fulfilling Amgen’s need of growth. -

Inozyme Pharma Expands Medical Leadership Team
Inozyme Pharma Expands Medical Leadership Team Strengthens Inozyme’s Ability to Advance Lead Candidate, INZ-701, into Clinical Trials Boston, Mass., Nov. 14, 2019 – Inozyme Pharma Inc., a biotechnology company developing novel medicines to treat rare and life-threatening mineralization disorders, today announced the addition of three industry veterans to its leadership team: • Pedro Huertas M.D., Ph.D., as Chief Medical Officer, • Gus Khursigara Ph.D., as Vice President of Medical Affairs and Clinical Operations, and • Catherine Nester, as Vice President of Physician and Patient Strategies. The executives will be instrumental in advancing Inozyme’s lead drug candidate, INZ-701, into clinical trials in 2020 for the treatment of patients with ENPP1 deficiency. INZ-701 is the first therapy that addresses the pathology of ENPP1 deficiency, including diseases such as generalized arterial calcification of infancy (GACI) type 1 and autosomal recessive hypophosphatemic rickets type 2 (ARHR2), both of which are rare and life-threatening manifestations of this enzyme deficiency. Inozyme Pharma received orphan drug designation for INZ-701 in the US and EU in 2018. “We are pleased and excited to welcome Pedro, Gus and Catherine to the Inozyme team,” said Axel Bolte, co-founder and chief executive officer of Inozyme. “Their expertise in orphan drug development – spanning medical affairs, regulatory affairs and clinical development – will strengthen and accelerate our ability to bring potentially life-saving medicines to people who urgently need effective treatments.” Dr. Huertas, a veteran of the pharmaceutical industry, has extensive experience in research and development and medical and regulatory affairs, especially regarding rare disorders and enzyme replacement therapies. -
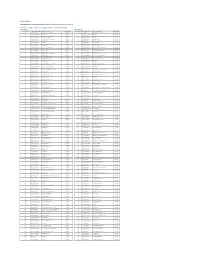
Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN)
Date: 20-Aug-21 Index Weights as of monthly rebalance date 10-Aug-21 Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN) Long Exposure Short Exposure Constituent Bloomberg Ticker Constituent Name Weight(%) Constituent Bloomberg Ticker Constituent Name Weight(%) 1 AAP UN Equity Advance Auto Parts Inc 0.24% 1 A UN Equity Agilent Technologies Inc -0.12% 2 ABBV UN Equity AbbVie Inc. 0.59% 2 HWM UN Equity Alcoa Inc -1.02% 3 ABC UN Equity AmerisourceBergen Corp 0.06% 3 AAL UW Equity American Airlines Group Inc -1.09% 4 ADBE UW Equity Adobe Systems Inc 0.01% 4 AAPL UW Equity Apple Inc. -0.46% 5 ADM UN Equity Archer-Daniels-Midland Co 0.26% 5 ABMD UW Equity ABIOMED Inc -0.11% 6 ADSK UW Equity Autodesk Inc 0.26% 6 ABT UN Equity Abbott Laboratories -0.26% 7 AES UN Equity AES Corp 0.37% 7 CB UN Equity ACE Limited -0.07% 8 AIG UN Equity American Intl Group Inc 0.52% 8 ACN UN Equity Accenture plc -0.29% 9 AIZ UN Equity Assurant Inc 0.11% 9 ADI UW Equity Analog Devices Inc -0.13% 10 ALGN UW Equity Align Technology Inc 0.59% 10 ADP UW Equity Automatic Data Processing -0.76% 11 ALL UN Equity Allstate Corp 0.16% 11 AEE UN Equity Ameren Corp -0.24% 12 ALLE UN Equity Allegion PLC 0.34% 12 AEP UW Equity American Electric Power -0.23% 13 AMAT UW Equity Applied Materials Inc 0.59% 13 AFL UN Equity AFLAC Inc -0.29% 14 AMD UW Equity Advanced Micro Devices Inc 1.15% 14 AJG UN Equity ARTHUR J GALLAGHER & CO -0.23% 15 AME UN Equity AMETEK Inc 0.26% 15 AKAM UW Equity Akamai Technologies Inc -0.11% 16 AMT UN Equity American Tower Corp A 0.39% 16 ALB UN -

Massmutual Mid Cap Growth Fund T
Fund Holdings As of 06/30/2021 MassMutual Mid Cap Growth Fund T. Rowe Price | Frontier Capital Prior to 5/1/2021, the Fund name was MassMutual Select Mid Cap Growth Fund. Fund Shares or Par Position Market Security Name Ticker CUSIP Weighting (%) Amount Value ($) Microchip Technology Inc MCHP 595017104 2.07 1,348,381 201,906,571 Agilent Technologies Inc A 00846U101 1.90 1,252,717 185,164,100 Hologic Inc HOLX 436440101 1.84 2,678,942 178,739,010 Teleflex Inc TFX 879369106 1.82 441,297 177,308,722 Ball Corp BLL 058498106 1.75 2,104,895 170,538,593 Textron Inc TXT 883203101 1.55 2,199,000 151,225,230 Burlington Stores Inc BURL 122017106 1.54 465,929 150,024,479 Catalent Inc CTLT 148806102 1.53 1,376,000 148,773,120 Marvell Technology Inc MRVL 573874104 1.46 2,442,368 142,463,325 Bruker Corp BRKR 116794108 1.39 1,776,000 134,940,480 Ingersoll Rand Inc IR 45687V106 1.29 2,564,000 125,148,840 The Cooper Companies Inc COO 216648402 1.28 314,645 124,684,374 KKR & Co Inc Ordinary Shares KKR 48251W104 1.25 2,050,813 121,490,162 Caesars Entertainment Inc CZR 12769G100 1.13 1,057,315 109,696,431 Reserve Invt Fds 0 76105Y208 1.07 103,877,547 103,877,547 DocuSign Inc DOCU 256163106 1.05 366,000 102,322,620 Chipotle Mexican Grill Inc CMG 169656105 1.04 65,283 101,210,846 Dollar General Corp DG 256677105 1.02 460,009 99,541,348 Veeva Systems Inc Class A VEEV 922475108 1.00 314,189 97,697,070 Avantor Inc AVTR 05352A100 0.98 2,700,000 95,877,000 JB Hunt Transport Services Inc JBHT 445658107 0.98 585,000 95,325,750 KLA Corp KLAC 482480100 0.97 291,710 94,575,299 -

CDER – Redi: Focus on CGMP & FDA Inspections – Participant List
FDA – CDER – RedI: Focus on CGMP & FDA Inspections – Participant List These participants granted permission to share their contact information Eileen Zhou Michael Channing A B M Mahfuz ul Alam Research Associate Group Chief - Positron Emission General Manager, Quality Operations Neuralstem Tomography Department ACI HealthCare Limited Germantown, MD, United States NIH Dhaka, Non-US, Bangladesh [email protected] Bethesda, MD, United States [email protected] [email protected] Abe Wong ABHIJEET GUJAR Adam Ebbinghouse CCO Chief Operating Officer QC Associate Gmpsigma SETHU KP Pharmaceutical Technology, Inc. Seattle, WA, United States Porvorim Goa, Non-US, India Bloomington, IN, United States [email protected] [email protected] [email protected] Adil Gatrad Adiseshu Modugula Aditiben Patel Director, Quality Systems RA Regulatory Specialist Actavis MSN USAMMDA Parsippany, NJ, United States Hyderabad, Non-US, India Frederick, MD, United States [email protected] [email protected] [email protected] Adriana de la Cruz ahsanul haque Aimee Gogarty Manager CSO Quality Coordinator Laboratorios Pisa SA de CV FDA Mallinckrodt Jalisco, Non-US, Mexico silver spring, MD, United States Port Allen, LA, United States [email protected] [email protected] [email protected] Aislyn Fronzak Ajay Deshmukh Ajay Khedkar QC Manager QA manager Regulatory Affair PL Developments Ingenus Pharmaceutical Umedica Laboratories Pvt Ltd Clinton, SC, United States Navi mumbai, Non-US, India Mumbai, Non-US, India [email protected] -

Companies in Attendance
COMPANIES IN ATTENDANCE Abbott Diabetes Care Archbow Consulting LLC Business One Technologies Abbott Laboratories ARKRAY USA BusinessOneTech AbbVie Armory Hill Advocates, LLC CastiaRX ACADIA Artia Solutions Catalyst Acaria Health Asembia Celgene Accredo Assertio Therapeutics Celltrion Acer Therapeutics AssistRx Center for Creative Leadership Acorda Therapeutics Astellas Pharma US Inc. Cigna Actelion AstraZeneca Cigna Specialty Pharmacy AdhereTech Athenex Oncology Circassia Advantage Point Solutions Avanir Coeus Consulting Group Aerie Pharmaceuticals Avella Coherus Biosciences AGIOS AveXis Collaborative Associates LLC Aimmune Theraputics Bank of America Collegium Akcea Therapeutics Bausch Health Corsica Life Sciences Akebia Therapeutics Bayer U.S. CoverMyMeds Alder BioPharmaceuticals Becton Dickinson Creehan & Company, Inc., an Inovalon Company Alexion Biofrontera CSL Behring Alkermes Biogen Curant Health Allergan Biohaven CVS Health Almirall BioMarin D2 Consulting Alnylam BioMatrix Specialty Pharmacy Daiichi Sankyo Amarin BioPlus Specialty Pharmacy DBV Technologies Amber Pharmacy Bioventus Deloitte Consulting LLP AmerisourceBergen Blue Cross Blue Shield Association Dendreon Amgen Blue Fin Group Dermira Amicus Therapeutics bluebird bio Dexcom Amneal Boehringer Ingelheim Diplomat Pharmacy Anthem Boston Biomedical Dova Applied Policy Bowler and Company Decision Resources Group Aquestive Therapeutics Braeburn Eisai Arbor Pharmaceuticals Bristol-Myers Squibb 1 electroCore Indivior Merz Pharmaceuticals EMD Serono Inside Rx Milliman Encore Dermatology, -

Mainstay WMC Enduring Capital Fund Q1 Holdings
MainStay MacKay Common Stock Fund Portfolio of Investments January 31, 2021† (Unaudited) Shares Value Common Stocks 98.2% Aerospace & Defense 2.0% Boeing Co. (The) 2,259 $ 438,675 Huntington Ingalls Industries, Inc. 5,380 846,435 Lockheed Martin Corp. 1,121 360,760 Northrop Grumman Corp. 342 98,021 Raytheon Technologies Corp. 6,466 431,476 Textron, Inc. 13,660 618,252 2,793,619 Air Freight & Logistics 0.7% FedEx Corp. 2,381 560,345 United Parcel Service, Inc., Class B 2,108 326,740 887,085 Auto Components 0.1% Aptiv plc 1,149 153,506 Automobiles 1.5% (a) Tesla, Inc. 2,563 2,033,817 Banks 3.6% Bank of America Corp. 14,882 441,251 Comerica, Inc. 14,415 824,538 Fifth Third Bancorp 19,190 555,167 JPMorgan Chase & Co. 5,995 771,377 Signature Bank 5,330 880,463 Synovus Financial Corp. 26,791 996,625 Truist Financial Corp. 10,429 500,383 4,969,804 Beverages 0.2% Coca-Cola Co. (The) 150 7,222 Molson Coors Beverage Co., Class B 3,023 151,634 PepsiCo, Inc. 897 122,503 281,359 Biotechnology 4.4% AbbVie, Inc. 6,231 638,553 Amgen, Inc. 5,750 1,388,222 Biogen, Inc. (a) 4,125 1,165,766 Exelixis, Inc. (a) 2,353 52,260 Gilead Sciences, Inc. 20,056 1,315,674 Incyte Corp. (a) 4,144 371,924 Regeneron Pharmaceuticals, Inc. (a) 751 378,384 United Therapeutics Corp. (a) 4,636 759,470 6,070,253 Building Products 0.6% Carrier Global Corp. -
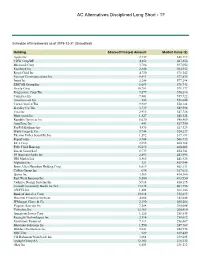
AC Alternatives Disciplined Long Short - TF
AC Alternatives Disciplined Long Short - TF Schedule of Investments as of 2019-12-31 (Unaudited) Holding Shares/Principal Amount Market Value ($) Apple Inc 2,139 628,117 CDW Corp/DE 4,252 607,356 Microsoft Corp 3,786 597,052 Facebook Inc 2,848 584,552 Royal Gold Inc 4,730 578,242 Verizon Communications Inc 9,411 577,835 Intuit Inc 2,204 577,294 EMCOR Group Inc 6,683 576,743 Oracle Corp 10,781 571,177 Progressive Corp/The 7,877 570,216 Comerica Inc 7,801 559,722 Amazon.com Inc 302 558,048 Coca-Cola Co/The 9,939 550,124 Hershey Co/The 3,739 549,558 Visa Inc 2,915 547,728 Mastercard Inc 1,827 545,524 Republic Services Inc 6,030 540,469 AutoZone Inc 443 527,750 PayPal Holdings Inc 4,875 527,329 Wells Fargo & Co 9,744 524,227 Thermo Fisher Scientific Inc 1,592 517,193 PepsiCo Inc 3,708 506,772 KLA Corp 2,695 480,168 Fifth Third Bancorp 15,615 480,005 Essent Group Ltd 8,739 454,341 PS Business Parks Inc 2,693 443,995 IHS Markit Ltd 5,865 441,928 Alphabet Inc 321 429,944 Booz Allen Hamilton Holding Corp 6,019 428,131 CoStar Group Inc 698 417,613 Qorvo Inc 3,565 414,360 East West Bancorp Inc 8,500 413,950 Cadence Design Systems Inc 5,914 410,195 United Community Banks Inc/GA 13,075 403,756 ANSYS Inc 1,403 361,146 Bank of America Corp 10,014 352,693 Discover Financial Services 4,068 345,048 JPMorgan Chase & Co 2,190 305,286 Flagstar Bancorp Inc 7,584 290,088 Valvoline Inc 12,163 260,410 American Tower Corp 1,125 258,548 Keysight Technologies Inc 2,514 258,012 Synchrony Financial 7,111 256,067 Motorola Solutions Inc 1,550 249,767 Builders FirstSource -

Wilmington Funds Holdings Template DRAFT
Wilmington Large-Cap Strategy Fund as of 5/31/2021 (Portfolio composition is subject to change) ISSUER NAME % OF ASSETS APPLE INC 4.97% MICROSOFT CORP 4.69% AMAZON.COM INC 3.45% FACEBOOK INC 1.99% ALPHABET INC 1.80% ALPHABET INC 1.77% BERKSHIRE HATHAWAY INC 1.48% JPMORGAN CHASE & CO 1.35% TESLA INC 1.20% JOHNSON & JOHNSON 1.12% UNITEDHEALTH GROUP INC 0.98% VISA INC 0.96% NVIDIA CORP 0.96% BANK OF AMERICA CORP 0.89% HOME DEPOT INC/THE 0.87% WALT DISNEY CO/THE 0.82% MASTERCARD INC 0.80% PAYPAL HOLDINGS INC 0.77% EXXON MOBIL CORP 0.68% PROCTER & GAMBLE CO/THE 0.67% COMCAST CORP 0.66% ADOBE INC 0.62% INTEL CORP 0.59% VERIZON COMMUNICATIONS INC 0.59% CISCO SYSTEMS INC 0.57% CHEVRON CORP 0.55% PFIZER INC 0.54% NETFLIX INC 0.54% AT&T INC 0.53% SALESFORCE.COM INC 0.53% ABBOTT LABORATORIES 0.51% ABBVIE INC 0.50% MERCK & CO INC 0.48% WELLS FARGO & CO 0.48% BROADCOM INC 0.47% THERMO FISHER SCIENTIFIC INC 0.47% ACCENTURE PLC 0.46% CITIGROUP INC 0.45% MCDONALD'S CORP 0.44% TEXAS INSTRUMENTS INC 0.44% COCA-COLA CO/THE 0.44% HONEYWELL INTERNATIONAL INC 0.44% LINDE PLC 0.43% MEDTRONIC PLC 0.43% NIKE INC 0.43% ELI LILLY & CO 0.42% PEPSICO INC 0.42% UNITED PARCEL SERVICE INC 0.41% WALMART INC 0.40% DANAHER CORP 0.40% UNION PACIFIC CORP 0.40% QUALCOMM INC 0.38% BRISTOL-MYERS SQUIBB CO 0.37% ORACLE CORP 0.37% LOWE'S COS INC 0.36% BLACKROCK INC 0.35% CATERPILLAR INC 0.35% AMGEN INC 0.35% BOEING CO/THE 0.35% MORGAN STANLEY 0.35% COSTCO WHOLESALE CORP 0.35% RAYTHEON TECHNOLOGIES CORP 0.34% STARBUCKS CORP 0.34% GOLDMAN SACHS GROUP INC/THE 0.34% GENERAL ELECTRIC -

Mainstay WMC Growth Fund Q1 Holdings
MainStay MacKay Growth Fund Portfolio of Investments January 31, 2021† (Unaudited) Shares Value Common Stocks 99.9% Aerospace & Defense 0.1% Huntington Ingalls Industries, Inc. 3,791 $ 596,438 Air Freight & Logistics 0.6% United Parcel Service, Inc., Class B 32,175 4,987,125 Automobiles 3.2% (a) Tesla, Inc. 32,810 26,035,719 Beverages 0.3% Coca-Cola Co. (The) 17,778 856,011 PepsiCo, Inc. 14,841 2,026,835 2,882,846 Biotechnology 5.7% AbbVie, Inc. 31,475 3,225,558 Alkermes plc (a) 125,474 2,633,699 Amgen, Inc. 45,353 10,949,575 Biogen, Inc. (a) 19,841 5,607,265 Exelixis, Inc. (a) 222,526 4,942,303 Gilead Sciences, Inc. 8,818 578,461 Incyte Corp. (a) 43,832 3,933,922 Moderna, Inc. (a) 12,659 2,192,032 Regeneron Pharmaceuticals, Inc. (a) 13,324 6,713,164 Seagen, Inc. (a) 12,671 2,081,465 United Therapeutics Corp. (a) 10,649 1,744,519 Vertex Pharmaceuticals, Inc. (a) 7,737 1,772,392 46,374,355 Capital Markets 0.8% Lazard Ltd., Class A 41,097 1,693,196 LPL Financial Holdings, Inc. 43,402 4,702,173 6,395,369 Construction & Engineering 0.6% Quanta Services, Inc. 65,450 4,612,261 Consumer Finance 0.5% SLM Corp. 322,393 4,474,815 Diversified Consumer Services 0.0%‡ Graham Holdings Co., Class B 691 392,564 Electronic Equipment, Instruments & Components 0.5% Jabil, Inc. 97,473 4,032,458 Shares Value Common Stocks Entertainment 2.0% (a) Lions Gate Entertainment Corp., Class B 263,020 $ 3,250,927 Netflix, Inc. -

2021 Jp Morgan Healthcare Conference
Cover page, paste image over entire page 2021 J.P. MORGAN HEALTHCARE CONFERENCE: DAY 1 January 2021|1 Summary The 39th annual J.P. Morgan Healthcare Conference (JPM) is being held virtually from January 11-14, 2021. A list of events and catalysts that were announced or updated at the conference today is included in this report. Below are some key points from today’s company presentations. Key Takeaways - Day 1 Mega Cap Companies • Amgen (AMGN) Chairman and CEO, Robert Bradway, started his presentation by focusing on Amgen’s two late stage assets, sotorasib and tezepelumab, at the J.P. Morgan Healthcare Conference given their large impact events expected this year, their potential to be first-in-class therapies, and the large unmet need they would be addressing in their indications. Key data catalysts for sotorasib in 2021 include a release of full Phase II results in KRAS G12C- mutant advanced NSCLC patients on January 29th at the World Lung Conference, Phase II colorectal cancer data in the first half of 2021, and initial data on several combinations with the KRAS inhibitor also in the first half of 2021. Regulatory submissions for sotorasib have been completed in the US and EU, and management indicates they are accelerating global launch preparations for sotorasib in anticipation of projected approvals this year. Successful approval would provide an effective targeted therapy option for previously treated NSCLC patients who have KRAS G12C-mutated locally advanced or metastatic, which account for 13% of the NSCLC patient population. In the long term, the Company indicates a possible exploration of label expansions to earlier lines of therapy and/or combinations with other therapies. -

2019 Annual Meeting Education Committee All Relationships Are Considered Compensated
2019 Annual Meeting Education Committee All relationships are considered compensated. Relationships are self-held unless otherwise noted. I = Immediate Family Member, Inst = My Institution Patents, Royalties, Travel, Stock and Other Expert Name Employment Leadership Honoraria Consulting or Advisory Role Speakers' Bureau Research Funding Other Intellectual Accommodations, Other Ownership Interests Testimony Property Expenses Monica M. Abbvie (Inst), Agenus (Inst), Astellas Bertagnolli Pharma (Inst), AstraZeneca (Inst), Baxalta (Inst), Bayer Health (Inst), Breast Cancer Research Foundation (Inst), Bristol- Myers Squibb (Inst), Celgene (Inst), Complion (Inst), Eisai (Inst), Exelixis (Inst), Genentech (Inst), GHI Pharma (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Janssen (Inst), Jazz Pharmaceuticals (Inst), Leidos (Inst), Lexicon (Inst), Lilly (Inst), Matrex (Inst), Mayo Clinic (Inst), Merck (Inst), MGH (Inst), Millenium Pharamceuticals (Inst), Novartis (Inst), PCORI (Inst), Pfizer (Inst), Pharmacyclics (Inst), Robert Wood Johnson Foundation (Inst), Sagerock Advisors (Inst), Sanofi (Inst), Taiho Oncology (Inst), Takeda (Inst), Tesaro (Inst), Teva (Inst) Tatiana M. Prowell David R. Spigel AstraZeneca (Inst), Boehringer AstraZeneca (Inst), Boehringer Ingelheim (Inst), Bristol-Myers Ingelheim (Inst), Bristol-Myers Squibb Squibb (Inst), Celgene (Inst), (Inst), Celgene (Inst), Genentech/Roche Genentech/Roche (Inst), (Inst), Lilly (Inst), Merck (Inst), Novartis Novartis (Inst), Pfizer (Inst) (Inst), Pfizer (Inst), University