2021 Jp Morgan Healthcare Conference
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prostate Cancer in Focus
PROSTATE CANCER IN FOCUS Current Developments in the Management of Prostate Cancer Section Editor: Andrew J. Armstrong, MD Prostate Cancer Prostate PSMA-Targeted Therapy in Prostate Cancer Scott T. Tagawa, MD, MS Professor of Medicine and Urology Weill Cornell Medicine New York, New York H&O What is prostate-specific membrane agent such as a monoclonal antibody might be able to antigen (PSMA), and what makes it a good target target a tumor without binding to other PSMA-positive for the treatment of prostate cancer? sites owing to its large size and physical inability to reach luminal sites of expression that are separated from the ST PSMA is a cell surface antigen that is expressed to vasculature by tight junctions. a limited degree on certain normal cells in the body but generally is highly overexpressed in the setting of prostate H&O How is PSMA positivity established? cancer. Normal cells that express PSMA are located in the prostate, salivary, and lacrimal glands; the proximal part ST Imaging and biopsy are the main ways to establish of the small intestine; the proximal renal tubules; and PSMA positivity. Multiple imaging modalities are avail- some ganglia. In the setting of prostate cancer, PSMA able worldwide. The agent indium In 111 capromab expression generally increases along with the cancer grade, pendetide (ProstaScint) has been approved for years in and it also increases following hormonal therapy. In addi- the United States, and the US Food and Drug Adminis- tion, PSMA expression tends to be higher in metastatic tration (FDA) approved the use of gallium Ga 68 PSMA- sites than in primary tumors. -

Amgen and Incyte – the Biotechnology Acquisition
Amgen and Incyte – the biotechnology acquisition Ana Carolina Coelho Student number: 152416013 Dissertation written under the supervision of António Luís Borges de Assunção Dissertation submitted in partial fulfilment of requirements for the MSc in Finance, at the Universidade Católica Portuguesa, May 2018 Abstract The main goal of this dissertation is to study the hypothesis of an acquisition in the biotechnology industry, between Amgen (acquirer) and Incyte (target). Amgen is a U.S.-based biotechnology company currently facing a decrease in sales, mainly due to an increase in the market share of generic drugs and the rise of biosimilar products. To offset the poor performance, it is looking for an acquisition in its industry, for which Incyte could be a suitable target. Incyte belongs to the U.S.-biotechnology industry, and although it has a shy presence in the market with only two released drugs, its revenues are expected to increase significantly in the near future. The combination of these companies would allow knowledge transfer about research and development process of new drugs, a stronger position in Europe, and a higher investment power to apply in R&D. The combined company would be able to decrease the number of employees due to duplication of jobs and decrease the cost of sales, as the power over suppliers increases. The potential synergies are valued at $28,522.8 million, of which $16,739.2 million are more likely to be realized. The combined firm, after the introduction of synergies, is expected to generate the same or even higher returns than the sum of the stand- alone businesses and higher growth rates, fulfilling Amgen’s need of growth. -

Inozyme Pharma Expands Medical Leadership Team
Inozyme Pharma Expands Medical Leadership Team Strengthens Inozyme’s Ability to Advance Lead Candidate, INZ-701, into Clinical Trials Boston, Mass., Nov. 14, 2019 – Inozyme Pharma Inc., a biotechnology company developing novel medicines to treat rare and life-threatening mineralization disorders, today announced the addition of three industry veterans to its leadership team: • Pedro Huertas M.D., Ph.D., as Chief Medical Officer, • Gus Khursigara Ph.D., as Vice President of Medical Affairs and Clinical Operations, and • Catherine Nester, as Vice President of Physician and Patient Strategies. The executives will be instrumental in advancing Inozyme’s lead drug candidate, INZ-701, into clinical trials in 2020 for the treatment of patients with ENPP1 deficiency. INZ-701 is the first therapy that addresses the pathology of ENPP1 deficiency, including diseases such as generalized arterial calcification of infancy (GACI) type 1 and autosomal recessive hypophosphatemic rickets type 2 (ARHR2), both of which are rare and life-threatening manifestations of this enzyme deficiency. Inozyme Pharma received orphan drug designation for INZ-701 in the US and EU in 2018. “We are pleased and excited to welcome Pedro, Gus and Catherine to the Inozyme team,” said Axel Bolte, co-founder and chief executive officer of Inozyme. “Their expertise in orphan drug development – spanning medical affairs, regulatory affairs and clinical development – will strengthen and accelerate our ability to bring potentially life-saving medicines to people who urgently need effective treatments.” Dr. Huertas, a veteran of the pharmaceutical industry, has extensive experience in research and development and medical and regulatory affairs, especially regarding rare disorders and enzyme replacement therapies. -

Victory Fund Holdings Victory Rs Mid Cap Growth
VICTORY FUND HOLDINGS As of June 30, 2021 VICTORY RS MID CAP GROWTH FUND MATURITY TRADED MARKET TRADED MARKET SECURITY DESCRIPTION DATE VALUE (BASE) VALUE (BASE) % ADVANCED DRAINAGE SYSTEMS INC 3,267,457.10 0.71% ALIGN TECHNOLOGY INC 6,788,210.00 1.48% AMERISOURCEBERGEN CORP. 4,443,356.90 0.97% APELLIS PHARMACEUTICALS INC 3,165,688.00 0.69% ARISTA NETWORKS INC 3,079,635.00 0.67% AVANTOR INC 4,718,923.90 1.03% AXON ENTERPRISE INC 6,244,576.00 1.37% BILL.COM HOLDINGS INC 4,132,540.80 0.90% BOSTON BEER COMPANY INC-CLASS A 3,521,760.00 0.77% BUILDERS FIRSTSOURCE INC 3,443,941.80 0.75% BURLINGTON STORES INC 5,383,672.80 1.18% CAESARS ENTERTAINMENT INC 4,181,125.00 0.91% CARMAX INC. 2,479,680.00 0.54% CARRIER GLOBAL CORP 5,655,096.00 1.24% CENTENE CORP. 3,567,079.23 0.78% CHARLES RIVER LABORATORIES INTERNATIONAL, INC. 8,030,963.20 1.76% CHART INDUSTRIES INC 3,985,756.80 0.87% CHEWY INC-CLASS A 5,215,106.46 1.14% CHIPOTLE MEXICAN GRILL, INC. 9,922,176.00 2.17% COSTAR GROUP INC 6,517,934.00 1.43% CROWDSTRIKE HOLDINGS INC-A 6,242,540.40 1.36% DARDEN RESTAURANTS, INC. 2,988,415.30 0.65% DEXCOM, INC. 5,435,710.00 1.19% DOCUSIGN INC 8,635,917.30 1.89% DOLBY LABORATORIES INC 4,895,824.90 1.07% DROPBOX INC 3,032,818.60 0.66% ENPHASE ENERGY INC 4,557,696.60 1.00% ENTEGRIS INC 7,171,610.40 1.57% FAIR ISAAC CORP. -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -
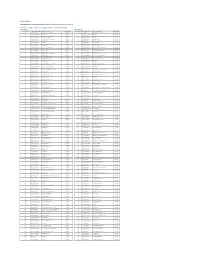
Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN)
Date: 20-Aug-21 Index Weights as of monthly rebalance date 10-Aug-21 Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN) Long Exposure Short Exposure Constituent Bloomberg Ticker Constituent Name Weight(%) Constituent Bloomberg Ticker Constituent Name Weight(%) 1 AAP UN Equity Advance Auto Parts Inc 0.24% 1 A UN Equity Agilent Technologies Inc -0.12% 2 ABBV UN Equity AbbVie Inc. 0.59% 2 HWM UN Equity Alcoa Inc -1.02% 3 ABC UN Equity AmerisourceBergen Corp 0.06% 3 AAL UW Equity American Airlines Group Inc -1.09% 4 ADBE UW Equity Adobe Systems Inc 0.01% 4 AAPL UW Equity Apple Inc. -0.46% 5 ADM UN Equity Archer-Daniels-Midland Co 0.26% 5 ABMD UW Equity ABIOMED Inc -0.11% 6 ADSK UW Equity Autodesk Inc 0.26% 6 ABT UN Equity Abbott Laboratories -0.26% 7 AES UN Equity AES Corp 0.37% 7 CB UN Equity ACE Limited -0.07% 8 AIG UN Equity American Intl Group Inc 0.52% 8 ACN UN Equity Accenture plc -0.29% 9 AIZ UN Equity Assurant Inc 0.11% 9 ADI UW Equity Analog Devices Inc -0.13% 10 ALGN UW Equity Align Technology Inc 0.59% 10 ADP UW Equity Automatic Data Processing -0.76% 11 ALL UN Equity Allstate Corp 0.16% 11 AEE UN Equity Ameren Corp -0.24% 12 ALLE UN Equity Allegion PLC 0.34% 12 AEP UW Equity American Electric Power -0.23% 13 AMAT UW Equity Applied Materials Inc 0.59% 13 AFL UN Equity AFLAC Inc -0.29% 14 AMD UW Equity Advanced Micro Devices Inc 1.15% 14 AJG UN Equity ARTHUR J GALLAGHER & CO -0.23% 15 AME UN Equity AMETEK Inc 0.26% 15 AKAM UW Equity Akamai Technologies Inc -0.11% 16 AMT UN Equity American Tower Corp A 0.39% 16 ALB UN -

Incyte Corporation 2016 Annual Report
2016 ANNUAL REPORT TABLE OF CONTENTS Letter to Shareholders 2 Key Planned Goals for 2017 6 Innovation 7 Growth 10 Strength 13 Corporate Responsibility 15 Company Information 19 ii Incyte’s Executive Management Team LETTER TO SHAREHOLDERS Standing, left to right: Steven H. Stein, MD; Dear Shareholders, Paula J. Swain; Wenqing Yao, PhD; Jonathan E. Dickinson; At Incyte, we believe that innovation and the discovery of new products David W. Gryska; creates long-term value for patients and society, as well as for our employ- Hervé Hoppenot; Michael Morrissey; ees and our shareholders. It is our commitment to these objectives that has Vijay Iyengar, MD enabled us to make significant progress in the last year. During 2016, we saw continued growth in the number of patients being treated with Jakafi® Seated, left to right: Eric H. Siegel, JD, MBA; (ruxolitinib), our JAK1/JAK2 inhibitor, and we also added Iclusig® (ponati- Reid M. Huber, PhD; nib) to our commercial portfolio as part of our European transaction with Barry P. Flannelly, PharmD, MBA ARIAD Pharmaceuticals, Inc. In February 2017, with Eli Lilly & Company, we announced the European approval of Olumiant® (baricitinib). I believe that we are on Summary of Revenue-Generating Products track to reach our goal of PRODUCT NAME DRUG NAME TARGET APPROVED TERRITORY INDICATION(S) becoming a world-class, Jakafi® ruxolitinib1 JAK1/JAK2 Global MF3; PV4 Jakavi® global biopharmaceutical organization. For the first Iclusig® ponatinib BCR-ABL Europe CML and Ph+ ALL5 time in the history of our company, Incyte’s total Olumiant® baricitinib2 JAK1/JAK2 Europe RA6 yearly revenue surpassed $1 billion in 2016. -

VIRTUS ZEVENBERGEN INNOVATIVE GROWTH STOCK FUND SCHEDULE of INVESTMENTS (Unaudited) MARCH 31, 2021
VIRTUS ZEVENBERGEN INNOVATIVE GROWTH STOCK FUND SCHEDULE OF INVESTMENTS (Unaudited) MARCH 31, 2021 ($ reported in thousands) Shares Value Shares Value Footnote Legend: (1) Non-income producing. COMMON STOCKS—99.0% Industrials—3.9% Communication Services—9.3% Desktop Metal, Inc. (1) Country Weightings† Coursera, Inc.(1) 7,100 $ 320 Class A 359,800 $ 5,361 (1) Uber Technologies, United States 87% Netflix, Inc. 84,500 44,080 (1) Snap, Inc. Class A(1) 586,650 30,676 Inc. 897,900 48,944 Canada 7 Zillow Group, Inc. 54,305 Brazil 5 Class C(1) 434,335 56,307 Israel 1 Information Technology—43.9% Total 100% 131,383 (1) Coupa Software, Inc. 78,450 19,964 † (1) % of total investments as of March 31, 2021. Consumer Discretionary—25.3% fuboTV, Inc. 315,350 6,976 (1) NVIDIA Corp. 107,000 57,131 Airbnb, Inc. Class A 91,100 17,121 (1) Amazon.com, Inc.(1) 15,200 47,030 Okta, Inc. 235,575 51,928 (1) Paylocity Holding Chegg, Inc. 322,325 27,610 (1) (1) Corp. 110,750 19,916 Chewy, Inc. Class A 340,400 28,835 (1) Fiverr International PayPal Holdings, Inc. 198,850 48,289 Ltd.(1) 27,650 6,005 QUALCOMM, Inc. 204,050 27,055 RingCentral, Inc. Lululemon Athletica, (1) (1) Class A 129,800 38,665 Inc. 41,690 12,787 (1) (1) ServiceNow, Inc. 78,325 39,171 MercadoLibre, Inc. 48,225 70,994 (1) Peloton Interactive, Inc. Shopify, Inc. Class A 78,200 86,528 (1) Snowflake, Inc. Class A 336,850 37,876 (1) Tesla, Inc.(1) 162,740 108,699 Class A 110,700 25,381 Sprout Social, Inc. -

Massmutual Mid Cap Growth Fund T
Fund Holdings As of 06/30/2021 MassMutual Mid Cap Growth Fund T. Rowe Price | Frontier Capital Prior to 5/1/2021, the Fund name was MassMutual Select Mid Cap Growth Fund. Fund Shares or Par Position Market Security Name Ticker CUSIP Weighting (%) Amount Value ($) Microchip Technology Inc MCHP 595017104 2.07 1,348,381 201,906,571 Agilent Technologies Inc A 00846U101 1.90 1,252,717 185,164,100 Hologic Inc HOLX 436440101 1.84 2,678,942 178,739,010 Teleflex Inc TFX 879369106 1.82 441,297 177,308,722 Ball Corp BLL 058498106 1.75 2,104,895 170,538,593 Textron Inc TXT 883203101 1.55 2,199,000 151,225,230 Burlington Stores Inc BURL 122017106 1.54 465,929 150,024,479 Catalent Inc CTLT 148806102 1.53 1,376,000 148,773,120 Marvell Technology Inc MRVL 573874104 1.46 2,442,368 142,463,325 Bruker Corp BRKR 116794108 1.39 1,776,000 134,940,480 Ingersoll Rand Inc IR 45687V106 1.29 2,564,000 125,148,840 The Cooper Companies Inc COO 216648402 1.28 314,645 124,684,374 KKR & Co Inc Ordinary Shares KKR 48251W104 1.25 2,050,813 121,490,162 Caesars Entertainment Inc CZR 12769G100 1.13 1,057,315 109,696,431 Reserve Invt Fds 0 76105Y208 1.07 103,877,547 103,877,547 DocuSign Inc DOCU 256163106 1.05 366,000 102,322,620 Chipotle Mexican Grill Inc CMG 169656105 1.04 65,283 101,210,846 Dollar General Corp DG 256677105 1.02 460,009 99,541,348 Veeva Systems Inc Class A VEEV 922475108 1.00 314,189 97,697,070 Avantor Inc AVTR 05352A100 0.98 2,700,000 95,877,000 JB Hunt Transport Services Inc JBHT 445658107 0.98 585,000 95,325,750 KLA Corp KLAC 482480100 0.97 291,710 94,575,299 -

CDER – Redi: Focus on CGMP & FDA Inspections – Participant List
FDA – CDER – RedI: Focus on CGMP & FDA Inspections – Participant List These participants granted permission to share their contact information Eileen Zhou Michael Channing A B M Mahfuz ul Alam Research Associate Group Chief - Positron Emission General Manager, Quality Operations Neuralstem Tomography Department ACI HealthCare Limited Germantown, MD, United States NIH Dhaka, Non-US, Bangladesh [email protected] Bethesda, MD, United States [email protected] [email protected] Abe Wong ABHIJEET GUJAR Adam Ebbinghouse CCO Chief Operating Officer QC Associate Gmpsigma SETHU KP Pharmaceutical Technology, Inc. Seattle, WA, United States Porvorim Goa, Non-US, India Bloomington, IN, United States [email protected] [email protected] [email protected] Adil Gatrad Adiseshu Modugula Aditiben Patel Director, Quality Systems RA Regulatory Specialist Actavis MSN USAMMDA Parsippany, NJ, United States Hyderabad, Non-US, India Frederick, MD, United States [email protected] [email protected] [email protected] Adriana de la Cruz ahsanul haque Aimee Gogarty Manager CSO Quality Coordinator Laboratorios Pisa SA de CV FDA Mallinckrodt Jalisco, Non-US, Mexico silver spring, MD, United States Port Allen, LA, United States [email protected] [email protected] [email protected] Aislyn Fronzak Ajay Deshmukh Ajay Khedkar QC Manager QA manager Regulatory Affair PL Developments Ingenus Pharmaceutical Umedica Laboratories Pvt Ltd Clinton, SC, United States Navi mumbai, Non-US, India Mumbai, Non-US, India [email protected] -

Fingolimod Rescues Demyelination in a Mouse Model of Krabbe's Disease
Research Articles: Neurobiology of Disease Fingolimod rescues demyelination in a mouse model of Krabbe's disease https://doi.org/10.1523/JNEUROSCI.2346-19.2020 Cite as: J. Neurosci 2020; 10.1523/JNEUROSCI.2346-19.2020 Received: 30 September 2019 Revised: 17 December 2019 Accepted: 21 January 2020 This Early Release article has been peer-reviewed and accepted, but has not been through the composition and copyediting processes. The final version may differ slightly in style or formatting and will contain links to any extended data. Alerts: Sign up at www.jneurosci.org/alerts to receive customized email alerts when the fully formatted version of this article is published. Copyright © 2020 Be´chet et al. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license, which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed. 1 Fingolimod rescues demyelination in a mouse model of Krabbe’s disease 2 Sibylle Béchet, Sinead O’Sullivan, Justin Yssel, Steven G. Fagan, Kumlesh K. Dev 3 Drug Development, School of Medicine, Trinity College Dublin, IRELAND 4 5 Corresponding author: Prof. Kumlesh K. Dev 6 Corresponding author’s address: Drug Development, School of Medicine, Trinity College 7 Dublin, IRELAND, D02 R590 8 Corresponding author’s phone and fax: Tel: +353 1 896 4180 9 Corresponding author’s e-mail address: [email protected] 10 11 Number of pages: 35 pages 12 Number of figures: 9 figures 13 Number of words (Abstract): 216 words 14 Number of words (Introduction): 641 words 15 Number of words (Discussion): 1475 words 16 17 Abbreviated title: Investigating the use of FTY720 in Krabbe’s disease 18 19 Acknowledgement statement (including conflict of interest and funding sources): This work 20 was supported, in part, by Trinity College Dublin, Ireland and the Health Research Board, 21 Ireland. -

Doctor of Pharmacy Fellowship Program
2021-2022 Doctor of Pharmacy Fellowship Program Accelerate your career with one of our 1-year fellowship programs Transformative Therapies Targeting Cancer Seagen Inc. is a global biotechnology company dedicated to discovering, developing, and commercializing transformative YEARS: In oncology for 23+ years cancer medicines to make a meaningful difference in people’s lives. SIZE: Largest biotechnology company ADCETRIS® (brentuximab vedotin) and PADCEV® (enfortumab vedotin-ejfv) use based in the Pacific Northwest the company’s industry-leading antibody-drug conjugate (ADC) technology. ADCETRIS is approved in certain CD30-expressing lymphomas, and PADCEV is EMPLOYEES: 2,200+ employees approved in certain metastatic urothelial cancers. TUKYSA® (tucatinib), a small mole- worldwide cule tyrosine kinase inhibitor, is approved in certain HER2-positive metastatic breast cancers. The company is headquartered in Seattle, Washington area, with loca- PASSION: Helping people with cancer tions in California, Switzerland and the European Union. Beyond our approved prod- ucts, the company has established a pipeline of novel targeted therapies at various stages of clinical testing. OUR MISSION OUR VALUES To discover, develop, and Passion for helping patients commercialize transformative cancer Revolutionizing therapies for people medicines to make a meaningful living with cancer difference in people’s lives. Integrity Honesty, respect and trust guide us Scientific excellence Premier science empowers our passion We have built a strong Diversity, teamwork, and corporate culture around our mutual respect mission and values. Seagen Shared dedication and diverse embodies an entrepreneurial perspectives drive successful spirit that advances collaborations breakthrough therapies, Innovation which is why we are the Entrepreneurial spirit advances leader in antibody-drug breakthrough therapies conjugate technology.