CDER – Redi: Focus on CGMP & FDA Inspections – Participant List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

By in Vivo's Biopharma, Medtech and Diagnostics Teams
invivo.pharmaintelligence.informa.com JANUARY 2018 Invol. 36 ❚ no. 01 Vivopharma intelligence ❚ informa 2018 OUTLOOK By In Vivo’s Biopharma, Medtech and Diagnostics Teams PAGE LEFT BLANK INTENTIONALLY invivo.pharmaintelligence.informa.com STRATEGIC INSIGHTS FOR LIFE SCIENCES DECISION-MAKERS CONTENTS ❚ In Vivo Pharma intelligence | January 2018 BIOPHARMA MEDTECH 2018 DIAGNOSTICS OUTLOOK 12 22 28 Biopharma 2018: Medtech 2018: Diagnostics 2018: Is There Still A Place For Pharma The Place For Innovation Steady Progress And In The New Health Care As Value-based Health Care The Big Get Bigger Economy? Gains Momentum MARK RATNER WILLIAM LOONEY ASHLEY YEO If the beginning of 2017 was marked 2018 will be a time of transition in health 2017 was a watershed year in many by doubts around whether and how care, when biopharma’s counterparts respects, politically, economically the FDA would act with respect to in adjacent industry segments scale up and commercially for many players complex diagnostics, we enter 2018 in a radical redesign of their traditional in the medtech field. Where will the feeling that slow-moving vessel may business models. Biopharma is not opportunities lie in 2018? Will finally be turning. moving as quickly, and it confronts a breakthrough medtech innovation still strategic dilemma on how to address the have a place among providers often prospect of a much more powerful set of riding on fumes when it comes to 36 rivals in the ongoing battle to own the budgets, and is it all as bad as some patient experience in medicine. would make out? Thirty-five Years Covering Health Care: The More Things Change… 30 PETER CHARLISH A Virtuous Cycle: What The The health care industry has come a Immuno-Oncology Revolution long way in the past 35 years, although Means For Other Disease Areas in some areas very little has changed. -

Amgen and Incyte – the Biotechnology Acquisition
Amgen and Incyte – the biotechnology acquisition Ana Carolina Coelho Student number: 152416013 Dissertation written under the supervision of António Luís Borges de Assunção Dissertation submitted in partial fulfilment of requirements for the MSc in Finance, at the Universidade Católica Portuguesa, May 2018 Abstract The main goal of this dissertation is to study the hypothesis of an acquisition in the biotechnology industry, between Amgen (acquirer) and Incyte (target). Amgen is a U.S.-based biotechnology company currently facing a decrease in sales, mainly due to an increase in the market share of generic drugs and the rise of biosimilar products. To offset the poor performance, it is looking for an acquisition in its industry, for which Incyte could be a suitable target. Incyte belongs to the U.S.-biotechnology industry, and although it has a shy presence in the market with only two released drugs, its revenues are expected to increase significantly in the near future. The combination of these companies would allow knowledge transfer about research and development process of new drugs, a stronger position in Europe, and a higher investment power to apply in R&D. The combined company would be able to decrease the number of employees due to duplication of jobs and decrease the cost of sales, as the power over suppliers increases. The potential synergies are valued at $28,522.8 million, of which $16,739.2 million are more likely to be realized. The combined firm, after the introduction of synergies, is expected to generate the same or even higher returns than the sum of the stand- alone businesses and higher growth rates, fulfilling Amgen’s need of growth. -

Biotech Investing 2020
BIOTECH INVESTING 2020 Bill Cara www.billcara.com [email protected] 647-868-6013 JULY 20, 2020 A. Report Overview This Greenfield Capital (billcara.com) report on the Biotechnology industry is written by a registered investment consultant & portfolio manager, not by a scientist. Our objective is to provide education and information to investors who lack the tools to trade successfully in an industry made vitally important to the world by the coronavirus pandemic of 2020. If we want to live through this pandemic -- and future ones -- where it is possible that hundreds of millions of us could perish because of the lack of diagnostics, vaccines, and therapeutics, then as investors we should be keenly interested in the Biotech industry. News reports state that in just six and a half months, COVID-19, initially called SARS- CoV-2, has killed more people than the number of Americans who die each year from opioid overdose (46,000), traffic accidents (36,500), and gun violence (40,000) combined. But, currently, there is no vaccine for COVID-19, and the treatment options for patients with severe or life-threatening symptoms are limited. As the world struggles to contain the deadly virus, there are, fortunately, over 140 coronavirus vaccines in development. News is breaking every day. At the same time, however, many individuals who are trading in Biotech stocks remain woefully uninformed and are speculating wildly. Some Biotech stocks that have appreciated 100% or more in a few months on hopefulness may have trouble sustaining gains beyond the news peak. Still, for investors who study this industry, there are opportunities right now to buy Biotech stocks that will grow in price well into the future. -

The Top 100 November, 2016 a List of Stocks Topping Our Custom 'Torpedo’ Screen
The Top 100 November, 2016 A list of stocks topping our custom 'torpedo’ screen. Updated monthly. GPRO GoPro, Inc. Class A Consumer Discretionary TSLA Tesla Motors, Inc. Consumer Discretionary UA Under Armour, Inc. Class A Consumer Discretionary CRC California Resources Corp Energy CRZO Carrizo Oil & Gas, Inc. Energy CWEI Clayton Williams Energy, Inc. Energy FANG Diamondback Energy, Inc. Energy GST Gastar Exploration, Inc. Energy MPLX MPLX LP Energy RSPP RSP Permian, Inc. Energy SLCA U.S. Silica Holdings, Inc. Energy AAC AAC Holdings, Inc. Health Care ABEO Abeona Therapeutics, Inc. Health Care ACAD ACADIA Pharmaceuticals Inc. Health Care ADMP Adamis Pharmaceuticals Corporation Health Care ADMS Adamas Pharmaceuticals, Inc. Health Care ADXS Advaxis, Inc. Health Care AIMT Aimmune Therapeutics Inc Health Care AKBA Akebia Therapeutics, Inc. Health Care ALDR Alder Biopharmaceuticals, Inc. Health Care ARDM Aradigm Corporation Health Care ARDX Ardelyx, Inc. Health Care ARLZ Aralez Pharmaceuticals Inc. Health Care ATRA Atara Biotherapeutics Inc Health Care BCRX BioCryst Pharmaceuticals, Inc. Health Care BLUE bluebird bio, Inc. Health Care CARA Cara Therapeutics Inc Health Care CDNA CareDx, Inc. Health Care CEMP Cempra, Inc. Health Care CERS Cerus Corporation Health Care CFMS ConforMIS Inc Health Care CLVS Clovis Oncology, Inc. Health Care COLL Collegium Pharmaceutical, Inc. Health Care CORI Corium International, Inc. Health Care CRMD CorMedix Inc. Health Care CSU Capital Senior Living Corporation Health Care DERM Dermira Inc Health Care DVAX Dynavax Technologies Corporation Health Care DXCM DexCom, Inc. Health Care EPZM Epizyme, Inc. Health Care FOLD Amicus Therapeutics, Inc. Health Care HRTX Heron Therapeutics Inc Health Care ICPT Intercept Pharmaceuticals, Inc. -

Inozyme Pharma Expands Medical Leadership Team
Inozyme Pharma Expands Medical Leadership Team Strengthens Inozyme’s Ability to Advance Lead Candidate, INZ-701, into Clinical Trials Boston, Mass., Nov. 14, 2019 – Inozyme Pharma Inc., a biotechnology company developing novel medicines to treat rare and life-threatening mineralization disorders, today announced the addition of three industry veterans to its leadership team: • Pedro Huertas M.D., Ph.D., as Chief Medical Officer, • Gus Khursigara Ph.D., as Vice President of Medical Affairs and Clinical Operations, and • Catherine Nester, as Vice President of Physician and Patient Strategies. The executives will be instrumental in advancing Inozyme’s lead drug candidate, INZ-701, into clinical trials in 2020 for the treatment of patients with ENPP1 deficiency. INZ-701 is the first therapy that addresses the pathology of ENPP1 deficiency, including diseases such as generalized arterial calcification of infancy (GACI) type 1 and autosomal recessive hypophosphatemic rickets type 2 (ARHR2), both of which are rare and life-threatening manifestations of this enzyme deficiency. Inozyme Pharma received orphan drug designation for INZ-701 in the US and EU in 2018. “We are pleased and excited to welcome Pedro, Gus and Catherine to the Inozyme team,” said Axel Bolte, co-founder and chief executive officer of Inozyme. “Their expertise in orphan drug development – spanning medical affairs, regulatory affairs and clinical development – will strengthen and accelerate our ability to bring potentially life-saving medicines to people who urgently need effective treatments.” Dr. Huertas, a veteran of the pharmaceutical industry, has extensive experience in research and development and medical and regulatory affairs, especially regarding rare disorders and enzyme replacement therapies. -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -
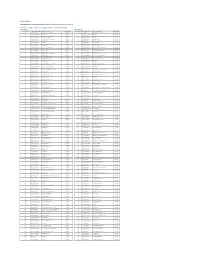
Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN)
Date: 20-Aug-21 Index Weights as of monthly rebalance date 10-Aug-21 Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN) Long Exposure Short Exposure Constituent Bloomberg Ticker Constituent Name Weight(%) Constituent Bloomberg Ticker Constituent Name Weight(%) 1 AAP UN Equity Advance Auto Parts Inc 0.24% 1 A UN Equity Agilent Technologies Inc -0.12% 2 ABBV UN Equity AbbVie Inc. 0.59% 2 HWM UN Equity Alcoa Inc -1.02% 3 ABC UN Equity AmerisourceBergen Corp 0.06% 3 AAL UW Equity American Airlines Group Inc -1.09% 4 ADBE UW Equity Adobe Systems Inc 0.01% 4 AAPL UW Equity Apple Inc. -0.46% 5 ADM UN Equity Archer-Daniels-Midland Co 0.26% 5 ABMD UW Equity ABIOMED Inc -0.11% 6 ADSK UW Equity Autodesk Inc 0.26% 6 ABT UN Equity Abbott Laboratories -0.26% 7 AES UN Equity AES Corp 0.37% 7 CB UN Equity ACE Limited -0.07% 8 AIG UN Equity American Intl Group Inc 0.52% 8 ACN UN Equity Accenture plc -0.29% 9 AIZ UN Equity Assurant Inc 0.11% 9 ADI UW Equity Analog Devices Inc -0.13% 10 ALGN UW Equity Align Technology Inc 0.59% 10 ADP UW Equity Automatic Data Processing -0.76% 11 ALL UN Equity Allstate Corp 0.16% 11 AEE UN Equity Ameren Corp -0.24% 12 ALLE UN Equity Allegion PLC 0.34% 12 AEP UW Equity American Electric Power -0.23% 13 AMAT UW Equity Applied Materials Inc 0.59% 13 AFL UN Equity AFLAC Inc -0.29% 14 AMD UW Equity Advanced Micro Devices Inc 1.15% 14 AJG UN Equity ARTHUR J GALLAGHER & CO -0.23% 15 AME UN Equity AMETEK Inc 0.26% 15 AKAM UW Equity Akamai Technologies Inc -0.11% 16 AMT UN Equity American Tower Corp A 0.39% 16 ALB UN -

Incyte Corporation 2016 Annual Report
2016 ANNUAL REPORT TABLE OF CONTENTS Letter to Shareholders 2 Key Planned Goals for 2017 6 Innovation 7 Growth 10 Strength 13 Corporate Responsibility 15 Company Information 19 ii Incyte’s Executive Management Team LETTER TO SHAREHOLDERS Standing, left to right: Steven H. Stein, MD; Dear Shareholders, Paula J. Swain; Wenqing Yao, PhD; Jonathan E. Dickinson; At Incyte, we believe that innovation and the discovery of new products David W. Gryska; creates long-term value for patients and society, as well as for our employ- Hervé Hoppenot; Michael Morrissey; ees and our shareholders. It is our commitment to these objectives that has Vijay Iyengar, MD enabled us to make significant progress in the last year. During 2016, we saw continued growth in the number of patients being treated with Jakafi® Seated, left to right: Eric H. Siegel, JD, MBA; (ruxolitinib), our JAK1/JAK2 inhibitor, and we also added Iclusig® (ponati- Reid M. Huber, PhD; nib) to our commercial portfolio as part of our European transaction with Barry P. Flannelly, PharmD, MBA ARIAD Pharmaceuticals, Inc. In February 2017, with Eli Lilly & Company, we announced the European approval of Olumiant® (baricitinib). I believe that we are on Summary of Revenue-Generating Products track to reach our goal of PRODUCT NAME DRUG NAME TARGET APPROVED TERRITORY INDICATION(S) becoming a world-class, Jakafi® ruxolitinib1 JAK1/JAK2 Global MF3; PV4 Jakavi® global biopharmaceutical organization. For the first Iclusig® ponatinib BCR-ABL Europe CML and Ph+ ALL5 time in the history of our company, Incyte’s total Olumiant® baricitinib2 JAK1/JAK2 Europe RA6 yearly revenue surpassed $1 billion in 2016. -

February 11-12, 2013 the Waldorf Astoria New York
February 11-12, 2013 The Waldorf Astoria New York 15th ANNU AL EVENT Now in its fifteenth year, the BIO CEO & Investor Conference is the largest independent investor conference focused on leading publicly-traded biotech companies. The meeting provides a neutral forum where institutional investors, industry analysts, and senior biotechnology executives have the opportunity to shape the future investment landscape of the biotechnology industry. Reasons Top 10 to attend 1 Present your company story to an audience of targeted investors. 2 Hear the Washington perspective on the Affordable Care Act, debt ceiling, and other timely policy developments affecting the industry. 3 Evaluate fresh investment opportunities including compatible, complementary and competitive companies. 4 Learn about the hottest clinical developments and industry catalysts by attending the conference’s therapeutic workshops and business roundtables. 5 Attend fireside chats with CEOs who will share their recent company successes, what keeps the C-suite up at night, and where the industry’s leading companies are headed in 2013. 6 Gain access to BIO’s 1x1 Partnering System for scouting potential deal partners and optimizing your time at the event. 7 Access presentations from more than 140 established public and private biotech companies and non-profit funding organizations, including many you won’t hear from at other investor conferences. 8 Get the pulse on the current and proposed investment trends in biotechnology. 9 Network with peers, investors and potential partners attending the conference. 10 It’s the first NYC biotech conference of the year, kicking off a week of key industry events that you don’t want to miss. -

Massmutual Mid Cap Growth Fund T
Fund Holdings As of 06/30/2021 MassMutual Mid Cap Growth Fund T. Rowe Price | Frontier Capital Prior to 5/1/2021, the Fund name was MassMutual Select Mid Cap Growth Fund. Fund Shares or Par Position Market Security Name Ticker CUSIP Weighting (%) Amount Value ($) Microchip Technology Inc MCHP 595017104 2.07 1,348,381 201,906,571 Agilent Technologies Inc A 00846U101 1.90 1,252,717 185,164,100 Hologic Inc HOLX 436440101 1.84 2,678,942 178,739,010 Teleflex Inc TFX 879369106 1.82 441,297 177,308,722 Ball Corp BLL 058498106 1.75 2,104,895 170,538,593 Textron Inc TXT 883203101 1.55 2,199,000 151,225,230 Burlington Stores Inc BURL 122017106 1.54 465,929 150,024,479 Catalent Inc CTLT 148806102 1.53 1,376,000 148,773,120 Marvell Technology Inc MRVL 573874104 1.46 2,442,368 142,463,325 Bruker Corp BRKR 116794108 1.39 1,776,000 134,940,480 Ingersoll Rand Inc IR 45687V106 1.29 2,564,000 125,148,840 The Cooper Companies Inc COO 216648402 1.28 314,645 124,684,374 KKR & Co Inc Ordinary Shares KKR 48251W104 1.25 2,050,813 121,490,162 Caesars Entertainment Inc CZR 12769G100 1.13 1,057,315 109,696,431 Reserve Invt Fds 0 76105Y208 1.07 103,877,547 103,877,547 DocuSign Inc DOCU 256163106 1.05 366,000 102,322,620 Chipotle Mexican Grill Inc CMG 169656105 1.04 65,283 101,210,846 Dollar General Corp DG 256677105 1.02 460,009 99,541,348 Veeva Systems Inc Class A VEEV 922475108 1.00 314,189 97,697,070 Avantor Inc AVTR 05352A100 0.98 2,700,000 95,877,000 JB Hunt Transport Services Inc JBHT 445658107 0.98 585,000 95,325,750 KLA Corp KLAC 482480100 0.97 291,710 94,575,299 -

Companies in Attendance
COMPANIES IN ATTENDANCE Abbott Diabetes Care Archbow Consulting LLC Business One Technologies Abbott Laboratories ARKRAY USA BusinessOneTech AbbVie Armory Hill Advocates, LLC CastiaRX ACADIA Artia Solutions Catalyst Acaria Health Asembia Celgene Accredo Assertio Therapeutics Celltrion Acer Therapeutics AssistRx Center for Creative Leadership Acorda Therapeutics Astellas Pharma US Inc. Cigna Actelion AstraZeneca Cigna Specialty Pharmacy AdhereTech Athenex Oncology Circassia Advantage Point Solutions Avanir Coeus Consulting Group Aerie Pharmaceuticals Avella Coherus Biosciences AGIOS AveXis Collaborative Associates LLC Aimmune Theraputics Bank of America Collegium Akcea Therapeutics Bausch Health Corsica Life Sciences Akebia Therapeutics Bayer U.S. CoverMyMeds Alder BioPharmaceuticals Becton Dickinson Creehan & Company, Inc., an Inovalon Company Alexion Biofrontera CSL Behring Alkermes Biogen Curant Health Allergan Biohaven CVS Health Almirall BioMarin D2 Consulting Alnylam BioMatrix Specialty Pharmacy Daiichi Sankyo Amarin BioPlus Specialty Pharmacy DBV Technologies Amber Pharmacy Bioventus Deloitte Consulting LLP AmerisourceBergen Blue Cross Blue Shield Association Dendreon Amgen Blue Fin Group Dermira Amicus Therapeutics bluebird bio Dexcom Amneal Boehringer Ingelheim Diplomat Pharmacy Anthem Boston Biomedical Dova Applied Policy Bowler and Company Decision Resources Group Aquestive Therapeutics Braeburn Eisai Arbor Pharmaceuticals Bristol-Myers Squibb 1 electroCore Indivior Merz Pharmaceuticals EMD Serono Inside Rx Milliman Encore Dermatology, -

Mainstay WMC Enduring Capital Fund Q1 Holdings
MainStay MacKay Common Stock Fund Portfolio of Investments January 31, 2021† (Unaudited) Shares Value Common Stocks 98.2% Aerospace & Defense 2.0% Boeing Co. (The) 2,259 $ 438,675 Huntington Ingalls Industries, Inc. 5,380 846,435 Lockheed Martin Corp. 1,121 360,760 Northrop Grumman Corp. 342 98,021 Raytheon Technologies Corp. 6,466 431,476 Textron, Inc. 13,660 618,252 2,793,619 Air Freight & Logistics 0.7% FedEx Corp. 2,381 560,345 United Parcel Service, Inc., Class B 2,108 326,740 887,085 Auto Components 0.1% Aptiv plc 1,149 153,506 Automobiles 1.5% (a) Tesla, Inc. 2,563 2,033,817 Banks 3.6% Bank of America Corp. 14,882 441,251 Comerica, Inc. 14,415 824,538 Fifth Third Bancorp 19,190 555,167 JPMorgan Chase & Co. 5,995 771,377 Signature Bank 5,330 880,463 Synovus Financial Corp. 26,791 996,625 Truist Financial Corp. 10,429 500,383 4,969,804 Beverages 0.2% Coca-Cola Co. (The) 150 7,222 Molson Coors Beverage Co., Class B 3,023 151,634 PepsiCo, Inc. 897 122,503 281,359 Biotechnology 4.4% AbbVie, Inc. 6,231 638,553 Amgen, Inc. 5,750 1,388,222 Biogen, Inc. (a) 4,125 1,165,766 Exelixis, Inc. (a) 2,353 52,260 Gilead Sciences, Inc. 20,056 1,315,674 Incyte Corp. (a) 4,144 371,924 Regeneron Pharmaceuticals, Inc. (a) 751 378,384 United Therapeutics Corp. (a) 4,636 759,470 6,070,253 Building Products 0.6% Carrier Global Corp.