Biotech Investing 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Top 100 November, 2016 a List of Stocks Topping Our Custom 'Torpedo’ Screen
The Top 100 November, 2016 A list of stocks topping our custom 'torpedo’ screen. Updated monthly. GPRO GoPro, Inc. Class A Consumer Discretionary TSLA Tesla Motors, Inc. Consumer Discretionary UA Under Armour, Inc. Class A Consumer Discretionary CRC California Resources Corp Energy CRZO Carrizo Oil & Gas, Inc. Energy CWEI Clayton Williams Energy, Inc. Energy FANG Diamondback Energy, Inc. Energy GST Gastar Exploration, Inc. Energy MPLX MPLX LP Energy RSPP RSP Permian, Inc. Energy SLCA U.S. Silica Holdings, Inc. Energy AAC AAC Holdings, Inc. Health Care ABEO Abeona Therapeutics, Inc. Health Care ACAD ACADIA Pharmaceuticals Inc. Health Care ADMP Adamis Pharmaceuticals Corporation Health Care ADMS Adamas Pharmaceuticals, Inc. Health Care ADXS Advaxis, Inc. Health Care AIMT Aimmune Therapeutics Inc Health Care AKBA Akebia Therapeutics, Inc. Health Care ALDR Alder Biopharmaceuticals, Inc. Health Care ARDM Aradigm Corporation Health Care ARDX Ardelyx, Inc. Health Care ARLZ Aralez Pharmaceuticals Inc. Health Care ATRA Atara Biotherapeutics Inc Health Care BCRX BioCryst Pharmaceuticals, Inc. Health Care BLUE bluebird bio, Inc. Health Care CARA Cara Therapeutics Inc Health Care CDNA CareDx, Inc. Health Care CEMP Cempra, Inc. Health Care CERS Cerus Corporation Health Care CFMS ConforMIS Inc Health Care CLVS Clovis Oncology, Inc. Health Care COLL Collegium Pharmaceutical, Inc. Health Care CORI Corium International, Inc. Health Care CRMD CorMedix Inc. Health Care CSU Capital Senior Living Corporation Health Care DERM Dermira Inc Health Care DVAX Dynavax Technologies Corporation Health Care DXCM DexCom, Inc. Health Care EPZM Epizyme, Inc. Health Care FOLD Amicus Therapeutics, Inc. Health Care HRTX Heron Therapeutics Inc Health Care ICPT Intercept Pharmaceuticals, Inc. -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

CDER – Redi: Focus on CGMP & FDA Inspections – Participant List
FDA – CDER – RedI: Focus on CGMP & FDA Inspections – Participant List These participants granted permission to share their contact information Eileen Zhou Michael Channing A B M Mahfuz ul Alam Research Associate Group Chief - Positron Emission General Manager, Quality Operations Neuralstem Tomography Department ACI HealthCare Limited Germantown, MD, United States NIH Dhaka, Non-US, Bangladesh [email protected] Bethesda, MD, United States [email protected] [email protected] Abe Wong ABHIJEET GUJAR Adam Ebbinghouse CCO Chief Operating Officer QC Associate Gmpsigma SETHU KP Pharmaceutical Technology, Inc. Seattle, WA, United States Porvorim Goa, Non-US, India Bloomington, IN, United States [email protected] [email protected] [email protected] Adil Gatrad Adiseshu Modugula Aditiben Patel Director, Quality Systems RA Regulatory Specialist Actavis MSN USAMMDA Parsippany, NJ, United States Hyderabad, Non-US, India Frederick, MD, United States [email protected] [email protected] [email protected] Adriana de la Cruz ahsanul haque Aimee Gogarty Manager CSO Quality Coordinator Laboratorios Pisa SA de CV FDA Mallinckrodt Jalisco, Non-US, Mexico silver spring, MD, United States Port Allen, LA, United States [email protected] [email protected] [email protected] Aislyn Fronzak Ajay Deshmukh Ajay Khedkar QC Manager QA manager Regulatory Affair PL Developments Ingenus Pharmaceutical Umedica Laboratories Pvt Ltd Clinton, SC, United States Navi mumbai, Non-US, India Mumbai, Non-US, India [email protected] -

11/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:25:21 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 11/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:25:21 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 01/01/2017 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -

Fidelity® Select Portfolio® Biotechnology Portfolio
Quarterly Holdings Report for Fidelity® Select Portfolio® Biotechnology Portfolio May 31, 2021 BIO-QTLY-0721 1.802156.117 Schedule of Investments May 31, 2021 (Unaudited) Showing Percentage of Net Assets Common Stocks – 97.4% Shares Value Shares Value Biotechnology – 89.4% CareDx, Inc. (a) 178,500 $ 14,351,400 Biotechnology – 89.4% Celldex Therapeutics, Inc. (a) 337,900 9,444,305 4D Molecular Therapeutics, Inc. 214,868 $ 5,706,894 Cellectis SA sponsored ADR (a) (b) 157,465 2,467,477 AbbVie, Inc. 9,829,284 1,112,674,949 Cerevel Therapeutics Holdings (a) 201,800 2,647,616 ACADIA Pharmaceuticals, Inc. (a) 199,594 4,458,930 ChemoCentryx, Inc. (a) 2,062,222 20,931,553 Acceleron Pharma, Inc. (a) 924,453 121,001,653 Chinook Therapeutics, Inc. (a) 840,646 13,870,659 ADC Therapeutics SA (a) (b) 755,238 16,350,903 Chinook Therapeutics, Inc. rights (a) (d) 115,821 5,791 Agios Pharmaceuticals, Inc. (a) 1,075,059 59,966,791 Codiak Biosciences, Inc. (b) 436,539 9,874,512 Akouos, Inc. (a) (b) 716,626 9,359,136 Coherus BioSciences, Inc. (a) 58,387 768,373 Albireo Pharma, Inc. (a) (b) 320,350 10,715,708 Connect Biopharma Holdings Ltd. ADR (a) 1,079,600 16,064,448 Aldeyra Therapeutics, Inc. (a) 1,201,911 15,047,926 Constellation Pharmaceuticals, Inc. (a) (b) 90,259 1,788,031 Alector, Inc. (a) (b) 998,482 17,772,980 ContraFect Corp. (a) (b) 456,309 1,843,488 Allakos, Inc. (a) 207,631 21,062,089 Cortexyme, Inc. -
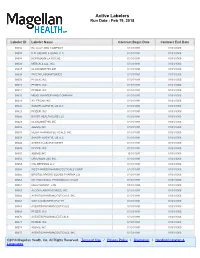
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

2016 Medicines in Development for Rare Diseases a LIST of ORPHAN DRUGS in the PIPELINE
2016 Medicines in Development for Rare Diseases A LIST OF ORPHAN DRUGS IN THE PIPELINE Autoimmune Diseases Product Name Sponsor Official FDA Designation* Development Status Actemra® Genentech treatment of systemic sclerosis Phase III tocilizumab South San Francisco, CA www.gene.com Adempas® Bayer HealthCare Pharmaceuticals treatment of systemic sclerosis Phase II riociguat Whippany, NJ www.pharma.bayer.com ARA 290 Araim Pharmaceuticals treatment of neuropathic pain in patients Phase II Tarrytown, NY with sarcoidosis www.ariampharma.com ARG201 arGentis Pharmaceuticals treatment of diffuse systemic sclerosis Phase II (type 1 native bovine skin Collierville, TN www.argentisrx.com collagen) BYM338 Novartis Pharmaceuticals treatment of inclusion body myositis Phase III (bimagrumab) East Hanover, NJ www.novartis.com CCX168 ChemoCentryx treatment of anti-neutrophil cytoplasmic Phase II (5a receptor antagonist) Mountain View, CA auto-antibodies associated vasculitides www.chemocentryx.com (granulomatosis with polyangitis or Wegener's granulomatosis), microscopic polyangitis, and Churg-Strauss syndrome * This designation is issued by the FDA's Office of Orphan Products Development while the drug is still in development. The designation makes the sponsor of the drug eligible for entitlements under the Orphan Drug Act of 1983. The entitlements include seven years of marketing exclusivity following FDA approval of the drug for the designated use. Medicines in Development: Rare Diseases | 2016 1 Autoimmune Diseases Product Name Sponsor Official FDA -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

BIOCRYST PHARMACEUTICALS, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K x Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the fiscal year ended December 31, 2005 OR o Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. For the transition period from ___________ to ___________. Commission File Number 000-23186 BIOCRYST PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) DELAWARE 62-1413174 (State of other jurisdiction of incorporation or organization) (I.R.S. employer identification no.) 2190 Parkway Lake Drive; Birmingham, Alabama 35244 (Address of principal executive offices) (205) 444-4600 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered None None Securities registered pursuant to Section 12(g) of the Act: Title of each class Common Stock, $.01 Par Value Indicate by a check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o Nox. Indicate by a check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o Nox. Indicate by a check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

BIOCRYST PHARMACEUTICALS, INC. 4505 Emperor Blvd., Suite 200 Durham, North Carolina 27703
BIOCRYST PHARMACEUTICALS, INC. 4505 Emperor Blvd., Suite 200 Durham, North Carolina 27703 NOTICE OF ANNUAL MEETING OF STOCKHOLDERS To Be Held June 20, 2018 To the Stockholders of BioCryst Pharmaceuticals, Inc.: Notice is hereby given that the Annual Meeting of Stockholders of BioCryst Pharmaceuticals, Inc., a Delaware corporation (the “Company”), will be held at our corporate offices at 4505 Emperor Blvd., Suite 200, Durham, NC 27703 on Wednesday, June 20, 2018 at 10:00 a.m., Eastern Daylight Time (the “Meeting”), for the following purposes: 1. To elect the two directors nominated in this Proxy Statement to serve for a term of three years and until a successor is duly elected and qualified; 2. To ratify the selection of Ernst & Young LLP as our independent registered public accountants for 2018; 3. To hold an advisory vote approving the Company’s executive compensation; 4. To transact such other business as may properly come before the Meeting or any adjournment thereof. THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT YOU VOTE IN FAVOR OF PROPOSALS 1, 2 AND 3. The proposals are further described in the Proxy Statement. The Board of Directors has fixed the close of business on May 2, 2018 as the record date for the determination of stockholders entitled to receive notice of and to vote at the Meeting or any adjournment thereof. The Meeting may be adjourned from time to time without notice other than announcement at the Meeting, and any business for which notice of the Meeting is hereby given may be transacted at any such adjournment. A list of the stockholders entitled to vote at the Meeting will be open to examination by any stockholder, for any purpose germane to the Meeting, during ordinary business hours, for a period of at least ten days prior to the Meeting at the principal executive offices of the Company in Durham, North Carolina. -

Biocryst Pharmaceuticals Inc
BIOCRYST PHARMACEUTICALS INC FORM 10-K (Annual Report) Filed 3/9/2006 For Period Ending 12/31/2005 Address 2190 PARKWAY LAKE DR BIRMINGHAM, Alabama 35244 Telephone 205-444-4600 CIK 0000882796 Industry Biotechnology & Drugs Sector Healthcare Fiscal Year 12/20 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the fiscal year ended December 31, 2005 OR Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. For the transition period from ___________ to ___________. Commission File Number 000-23186 BIOCRYST PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) DELAWARE 62 -1413174 (State of other jurisdiction of incorporation or organization) (I.R.S. employer identification no.) 2190 Parkway Lake Drive; Birmingham, Alabama 35244 (Address of principal executive offices) (205) 444-4600 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered None None Securities registered pursuant to Section 12(g) of the Act: Title of each class Common Stock, $.01 Par Value Indicate by a check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No . Indicate by a check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes No . Indicate by a check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
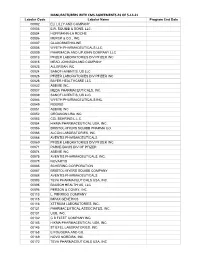
MANUFACTURERS with CMS AGREEMENTS AS of 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY and COMPANY 00003 E.R
MANUFACTURERS WITH CMS AGREEMENTS AS OF 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC Labeler Code Labeler Name Program End Date 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. 00186 ASTRAZENECA PHARMACEUTICALS LP 00187 BAUSCH HEALTH US, LLC. 00206 WYETH PHARMACEUTICALS LLC, 00224 KONSYL PHARMACEUTICALS, INC. 00225 B. F. ASCHER AND COMPANY, INC. 00228 ACTAVIS PHARMA, INC.