Reduction in the Risk of Major Adverse Cardiovascular Events
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kopi Af Aktivlisten 2021-06-30 Ny.Xlsm
Velliv noterede aktier i alt pr. 30-06-2021 ISIN Udstedelsesland Navn Markedsværdi (i DKK) US0378331005 US APPLE INC 1.677.392.695 US5949181045 US MICROSOFT CORP 1.463.792.732 US0231351067 US AMAZON.COM INC 1.383.643.996 DK0060534915 DK NOVO NORDISK A/S-B 1.195.448.146 US30303M1027 US FACEBOOK INC-CLASS A 1.169.094.867 US02079K3059 US ALPHABET INC-CL A 867.740.769 DK0010274414 DK DANSKE BANK A/S 761.684.457 DK0060079531 DK DSV PANALPINA A/S 629.313.827 US02079K1079 US ALPHABET INC-CL C 589.305.120 US90138F1021 US TWILIO INC - A 514.807.852 US57636Q1040 US MASTERCARD INC - A 490.766.560 US4781601046 US JOHNSON & JOHNSON 478.682.981 US70450Y1038 US PAYPAL HOLDINGS INC 471.592.728 DK0061539921 DK VESTAS WIND SYSTEMS A/S 441.187.698 US79466L3024 US SALESFORCE.COM INC 439.114.061 US01609W1027 US ALIBABA GROUP HOLDING-SP ADR 432.325.255 US8835561023 US THERMO FISHER SCIENTIFIC INC 430.036.612 US22788C1053 US CROWDSTRIKE HOLDINGS INC - A 400.408.622 KYG875721634 HK TENCENT HOLDINGS LTD 397.054.685 KR7005930003 KR SAMSUNG ELECTRONICS CO LTD 389.413.700 DK0060094928 DK ORSTED A/S 378.578.374 ES0109067019 ES AMADEUS IT GROUP SA 375.824.429 US46625H1005 US JPMORGAN CHASE & CO 375.282.618 US67066G1040 US NVIDIA CORP 357.034.119 US17275R1023 US CISCO SYSTEMS INC 348.160.692 DK0010244508 DK AP MOLLER-MAERSK A/S-B 339.783.859 US20030N1019 US COMCAST CORP-CLASS A 337.806.502 NL0010273215 NL ASML HOLDING NV 334.040.559 CH0012032048 CH ROCHE HOLDING AG-GENUSSCHEIN 325.008.200 KYG970081173 HK WUXI BIOLOGICS CAYMAN INC 321.300.236 US4370761029 US HOME DEPOT INC 317.083.124 US58933Y1055 US MERCK & CO. -

EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
December 22, 2008 EP Vantage Interview - Silence hoping to have something to shout about in 2009 Lisa Urquhart With its first product expected to go into the clinic potentially as early as January, Silence Therapeutics is hoping that 2009 will be year it has something to shout about and one that will reverse the alarming share price decline that has seen the company’s valuation slip from a high of over £170m in June 2007 to £21.6m today. Silence is one of a growing number of companies working in RNA interference (RNAi) and particularly short interfering RNA (siRNA), which work by selectively silencing or inactivating genes related to certain diseases. It is importantly one of only two companies that have composition of matter patent protection for their siRNA drugs. Speaking to EP Vantage, Iain Ross, chief executive of Silence, says: “It’s a big year coming up for us.” The group has spent most of the last 12 months strengthening its IP position, and next year comes the important move of the group’s lead candidate Atu027 into the clinic, an event Mr Ross says should take place in the first quarter of the year. Many expect it could be as soon as the end of January. Partnering focus What is less expected is the speed at which Mr Ross intends to partner the drug, which is being developed in solid tumours, with an emphasis on lung cancer. “We would be looking at doing something either at the end of 2009 or the beginning of 2010,” he says. Key to partnering discussions will be the drug demonstrating good safety data in a number of cancer patients, which could be the catalyst to starting talks with big pharma who have already started to ask about Atu027. -

IQVIA Pharma Deals Half-Year Review of 2020
White Paper IQVIA Pharma Deals Half-Year Review of 2020 HEATHER CARTWRIGHT, Senior Analyst, Global Market Insights, IQVIA MICHELLE LIU, Analyst, Global Market Insights, IQVIA TASKIN AHMED, Manager, Global Market Insights, IQVIA Table of contents Introduction 1 Uncertainty and prudence cause M&A to stall 2 Licensing deal values continue to rise 5 Roche pips AstraZeneca to title of most active dealmaker 10 COVID-19 pandemic drives increase in R&D collaboration 11 Infectious diseases replace oncology as top therapeutic area for dealmaking 14 Outlook for H2 2020 15 About the authors 17 Introduction COVID-19 shakes up the dealmaking landscape H1 2020 was an unprecedented time for dealmaking in the life sciences sector. While the COVID-19 pandemic rapidly provoked a significant level of partnering activity amongst biopharma companies keen to expedite the development of vaccines or therapeutics to tackle the virus, it also acted as a brake on other types of dealmaking, most notably M&A which saw a sharp decline in activity as a result of uncertainty and operational disruption. Indeed, H1 2020 was notable for the absence of any US$5 B deals on the scale seen in previous years as companies instead focused their attentions on internal programs and potential COVID-19 solutions, opting only for a few asset-driven acquisitions. There was also plentiful capital available to allow biotech companies to stay independent, particularly those in the US. As a result, aggregate spending on M&A by life science companies plummeted to just US$19.1 B in the first 6 months of 2020. -
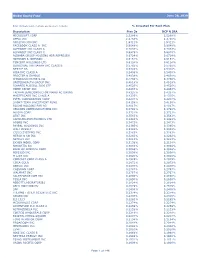
Global Equity Fund Description Plan 3S DCP & JRA MICROSOFT CORP
Global Equity Fund June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA MICROSOFT CORP 2.5289% 2.5289% APPLE INC 2.4756% 2.4756% AMAZON COM INC 1.9411% 1.9411% FACEBOOK CLASS A INC 0.9048% 0.9048% ALPHABET INC CLASS A 0.7033% 0.7033% ALPHABET INC CLASS C 0.6978% 0.6978% ALIBABA GROUP HOLDING ADR REPRESEN 0.6724% 0.6724% JOHNSON & JOHNSON 0.6151% 0.6151% TENCENT HOLDINGS LTD 0.6124% 0.6124% BERKSHIRE HATHAWAY INC CLASS B 0.5765% 0.5765% NESTLE SA 0.5428% 0.5428% VISA INC CLASS A 0.5408% 0.5408% PROCTER & GAMBLE 0.4838% 0.4838% JPMORGAN CHASE & CO 0.4730% 0.4730% UNITEDHEALTH GROUP INC 0.4619% 0.4619% ISHARES RUSSELL 3000 ETF 0.4525% 0.4525% HOME DEPOT INC 0.4463% 0.4463% TAIWAN SEMICONDUCTOR MANUFACTURING 0.4337% 0.4337% MASTERCARD INC CLASS A 0.4325% 0.4325% INTEL CORPORATION CORP 0.4207% 0.4207% SHORT-TERM INVESTMENT FUND 0.4158% 0.4158% ROCHE HOLDING PAR AG 0.4017% 0.4017% VERIZON COMMUNICATIONS INC 0.3792% 0.3792% NVIDIA CORP 0.3721% 0.3721% AT&T INC 0.3583% 0.3583% SAMSUNG ELECTRONICS LTD 0.3483% 0.3483% ADOBE INC 0.3473% 0.3473% PAYPAL HOLDINGS INC 0.3395% 0.3395% WALT DISNEY 0.3342% 0.3342% CISCO SYSTEMS INC 0.3283% 0.3283% MERCK & CO INC 0.3242% 0.3242% NETFLIX INC 0.3213% 0.3213% EXXON MOBIL CORP 0.3138% 0.3138% NOVARTIS AG 0.3084% 0.3084% BANK OF AMERICA CORP 0.3046% 0.3046% PEPSICO INC 0.3036% 0.3036% PFIZER INC 0.3020% 0.3020% COMCAST CORP CLASS A 0.2929% 0.2929% COCA-COLA 0.2872% 0.2872% ABBVIE INC 0.2870% 0.2870% CHEVRON CORP 0.2767% 0.2767% WALMART INC 0.2767% -

Unlocking the Potential of Gene Silencing for Everyone
Corporate Presentation JP Morgan Conference January 2018 Forward looking statements The information contained in this presentation is being supplied and communicated to you on a Securities in the Company have not been, and will not be, registered under the United States confidential basis solely for your information and may not be reproduced, further distributed to Securities Act of 1933, as amended (the “Securities Act”), or qualified for sale under the law of any other person or published, in whole or in part, for any purpose. In accordance with the any state or other jurisdiction of the United States of America and may not be offered or sold in prohibition on market abuse contained in Part VIII of the Financial Services and Markets Act the United States of America except pursuant to an exemption from, or in a transaction not 2000 (as amended) (the “Act”): (i) you must not pass this information to any person; and (ii) subject to, the registration requirements of the Securities Act. Neither the United States you must not base any behaviour in relation to any securities or other Qualifying Investments Securities and Exchange Commission nor any securities regulatory body of any state or other (as that term is defined in the Act) which would amount to market abuse on such information jurisdiction of the United States of America, nor any securities regulatory body of any other until after it is made generally available. country or political subdivision thereof, has approved or disapproved of this presentation or the securities discussed herein or passed on the accuracy or adequacy of the contents of this This presentation is being communicated in the United Kingdom only to (a) persons who have presentation. -

GSK's Big Reveal
3 August 2018 No. 3916 Scripscrip.pharmaintelligence.informa.com Pharma intelligence | informa at Genentech Inc. (Also see “GSK Bags Bar- ron As R&D Boss As Vallance Joins UK Govern- ment” - Scrip, 8 Nov, 2017.) With a budding early immuno-oncology pipeline, Walmsley clearly has her eye on establishing GSK as an important player in the space. The event in London was Barron’s first op- portunity to present his R&D strategy and coincided with the company’s second quar- ter sales and earnings release. “This is an ideal time to be thinking about reinventing R&D, because we are doing well. We are growing,” Barron said. PILLARS FOR INNOVATION Investors have been eager to hear from the R&D legend, but with few near-term catalysts or big changes to the late-stage pipeline, they may be underwhelmed by the update. Barron, meanwhile, is focused on deliver- GSK’s Big Reveal: ing scientific and cultural changes that will yield breakthroughs over the long-term. An R&D Overhaul Poised To Yield He talked about a time horizon of 2021 to 2026, and he talked more high-level, rather than specifics, about incentives needed to Long-Term Cultural Change drive innovation, the technologies that can JESSICA MERRILL [email protected] help improve clinical trial success, and cul- tural changes. He said science, technology laxoSmithKline PLC CEO Emma pharma R&D – is that investors will need to and culture are the three pillars needed to Walmsley may have spelled out the be patient to see GSK deliver on its promise drive innovation, and all three must be in Gbig pharma’s R&D turnaround plan of innovation. -

2019 Annual Meeting Education Committee All Relationships Are Considered Compensated
2019 Annual Meeting Education Committee All relationships are considered compensated. Relationships are self-held unless otherwise noted. I = Immediate Family Member, Inst = My Institution Patents, Royalties, Travel, Stock and Other Expert Name Employment Leadership Honoraria Consulting or Advisory Role Speakers' Bureau Research Funding Other Intellectual Accommodations, Other Ownership Interests Testimony Property Expenses Monica M. Abbvie (Inst), Agenus (Inst), Astellas Bertagnolli Pharma (Inst), AstraZeneca (Inst), Baxalta (Inst), Bayer Health (Inst), Breast Cancer Research Foundation (Inst), Bristol- Myers Squibb (Inst), Celgene (Inst), Complion (Inst), Eisai (Inst), Exelixis (Inst), Genentech (Inst), GHI Pharma (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Janssen (Inst), Jazz Pharmaceuticals (Inst), Leidos (Inst), Lexicon (Inst), Lilly (Inst), Matrex (Inst), Mayo Clinic (Inst), Merck (Inst), MGH (Inst), Millenium Pharamceuticals (Inst), Novartis (Inst), PCORI (Inst), Pfizer (Inst), Pharmacyclics (Inst), Robert Wood Johnson Foundation (Inst), Sagerock Advisors (Inst), Sanofi (Inst), Taiho Oncology (Inst), Takeda (Inst), Tesaro (Inst), Teva (Inst) Tatiana M. Prowell David R. Spigel AstraZeneca (Inst), Boehringer AstraZeneca (Inst), Boehringer Ingelheim (Inst), Bristol-Myers Ingelheim (Inst), Bristol-Myers Squibb Squibb (Inst), Celgene (Inst), (Inst), Celgene (Inst), Genentech/Roche Genentech/Roche (Inst), (Inst), Lilly (Inst), Merck (Inst), Novartis Novartis (Inst), Pfizer (Inst) (Inst), Pfizer (Inst), University -

SILENCE THERAPEUTICS PLC (Exact Name of Registrant As Specified in Its Charter)
Table of Contents As filed with the Securities and Exchange Commission on April 8, 2021 Registration No. 333-248203 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Post-Effective Amendment No. 2 to Form F-1 REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933 SILENCE THERAPEUTICS PLC (Exact Name of Registrant as Specified in its Charter) Not Applicable (Translation of Registrant’s Name into English) England and Wales 2834 Not Applicable (State or other Jurisdiction of (Primary Standard Industrial (I.R.S. Employer Incorporation or Organization) Classification Code Number) Identification Number) 72 Hammersmith Road London W14 8TH United Kingdom Tel: +44 20 3457 6900 (Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices) Silence Therapeutics Inc. 434 West 33rd Street, Office 814 New York, New York 10001 Tel: +1 917 374 0372 (Name, address, including zip code, and telephone number, including area code, of agent for service) Copies of all communications, including communications sent to agent for service, should be sent to: Joshua A. Kaufman Claire A. Keast-Butler Divakar Gupta Cooley (UK) LLP Brian F. Leaf Dashwood Cooley LLP 69 Old Broad Street 55 Hudson Yards London EC2M 1QS New York, New York 10001 United Kingdom +1 212 479—6000 +44 20 7785 9355 Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. -

Silence Therapeutics
THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION. If you are in any doubt about the contents of this document and the action you should take, you are recommended immediately to seek your own advice from a person duly authorised under the Financial Services and Markets Act 2000 (as amended) who specialises in advising on the acquisition of shares and other securities. If you have sold or otherwise transferred all of your Ordinary Shares in Silence Therapeutic s plc or prior to 15 December 2009 a sale or transfer is effected, please send this document, together with the Form of Proxy, to the purchaser or transferee or to the stockbroker, bank or other agent through whom the sale or transfer was effected. This document constitutes an admission document drawn up in accordance with the AIM Rules for Companies and does not comprise a prospectus prepared in accordance with the Prospectus Rules of the UK Listing Authority made under section 73A of FSMA, and it has not been, and will not be, approved or filed with the Financial Services Authority under the Prospectus Rules. The Directors and the Proposed Directors , whose names appear on page 3 of this document, accept individual and collective responsibility for the information contained in this document including individual and collective responsibility for compliance with the AIM Rules. To the best of the knowledge of the Directors and the Proposed Directors (who have taken all reasonable care to ensure that such is the case), the information contained in this document is in accordance with the facts and contains no omission likely to affect the import of such information. -

Audrey's Life Science Meeting Picks for December-January 2011
Audrey’s Life Science Meeting Picks for December-January 2011 Complimentary Service of AudreysNetwork.com (Dec. 5, 2011 Edition) ******************************************************************************* BioCentury TV Today, See the Webcast Sunday, Dec. 4, 2011 www.biocenturytv.com, Continuously available starting at 9:00 a.m. EDT Topic: “The Doctor in the House” Editor Steve Usdin is joined by Michael Burgess, Republican Congressional Representative from Texas Date: Sunday, Dec. 4, 2011 Watch the Broadcast Watch on the Web 8:30 - 9:00 a.m. EDT www.biocenturytv.com WUSA Channel 9 Continuously available in Washington, D.C. starting at 9:00 a.m. Topic Description Representative Michael Burgess was an obstetrician for 25 years before being sent to Congress from Texas. Now vice-chair of the House subcommittee on health, he will be among the first lawmakers put his stamp on the must-pass user fee legislation for drugs and devices in 2012. On Sunday, December 4, BioCentury This Week Washington Editor Steve Usdin and Rep. Burgess discuss why the conservative Texas Republican doesn't see eye to eye with the White House on healthcare reform, why he wants to limit FDA's oversight of lab-developed tests, and his efforts to relax financial conflict of interest restrictions on the agency's advisory committee members. ************************************************ Bio2Device Group, Tuesday Morning, Dec. 6, 2011 Topic: Title: "What's So Complicated About Medical Customer Support? - Views From Behind the Curtains" Panelists: John Jensen, Ron Bucher, Stacy Williams Date and Time: Tuesday, Dec. 6, 2011, 8:30- 10:30 am Location: Sunnyvale City Council Chambers, 456 W. Olive, Sunnyvale, CA (across the street from Sunnyvale Public Library) Cost: Free Park in Street and in NOVA and library parking lots across the street. -

DDT Cover/Back April 2006.Qx
April 2008 Vol 8 No 4 www.drugdeliverytech.com IN THIS ISSUE INTERVIEW WITH PHARMAFORM’S VP, BUSINESS DEVELOPMENT MICHAEL CROWLEY, PhD MBO Financing 28 Derek G. Hennecke, MBA Injectable Packaging 40 Frances L. DeGrazio Partnering With dermaCM 56 Robert L. Dowdell FEATURING CRO Strategies 60 Cindy H. Dubin Late-Stage The science & business of specialty pharma, biotechnology, and drug delivery Development 68 Robert Zerbe, MD Bhupendra Christine M. Stuart Madden, Prajapati, Ford, MBA Challenges in MPharm Investment Trends PhD Driving Combination Which Way to 78 The Upcoming Era China Product Drug Approval? Ames Gross of Nanomedicine: A Development Briefing April 2008 Vol 8 No 4 PUBLISHER/PRESIDENT Ralph Vitaro EXECUTIVE EDITORIAL DIRECTOR Dan Marino, MSc [email protected] CREATIVE DIRECTOR Shalamar Q. Eagel CONTROLLER Debbie Carrillo CONTRIBUTING EDITORS Cindy H. Dubin Debra Bingham Jason McKinnie Malcolm A. Teasdale TECHNICAL OPERATIONS Mark Newland EDITORIAL SUPPORT Nicholas D. Vitaro ADMINISTRATIVE SUPPORT Kathleen Kenny Corporate/Editorial Office 219 Changebridge Road, Montville, NJ 07045 Tel: (973)299-1200 Fax: (973) 299-7937 www.drugdeliverytech.com Advertising Sales Offices East & Midwest Victoria Geis - Account Executive Cheryl S. Stratos - Account Executive 103 Oronoco Street, Suite 200 Alexandria, VA 22314 Tel: (703) 212-7735 Fax: (703) 548-3733 E-mail: [email protected] E-mail: [email protected] West Coast Warren De Graff Western Regional Manager The bi-weekly electronic newsletter from the publishers of Drug 818 5th Avenue, Suite 301 San Rafael, CA 94901 Delivery Technology and Specialty Pharma will provide over 12,000 Tel: (415) 721-0644 Fax: (415) 721-0665 subscribers with the latest news of business deals, alliances, and E-mail: [email protected] technology breakthroughs from the pharmaceutical, specialty International Ralph Vitaro pharmaceutical, drug delivery, and biotechnology industries. -

TC 1600 Pgrs.Xlsx
Drug (if Trial Patent No. Filed Date Lead Pet. Patent Owner(s) Inst. Dec. Outcome Details of FWD applicable) <Information obtained primarily from Lex Machina™ and public sources on the Internet. If any errors are identified, please contact Lisa Mandrusiak at Oblon.> Roche Palo Alto LLC; Helsinn PGR2014-00010 Aloxi® 8,598,219 2-Sep-14 Accord Healthcare, Inc. Healthcare S.A. N/A Settled pre-institution N/A Phenyl-ephrine PGR2015-00011 HCl 8,859,623 11-May-15 Altaire Pharmaceuticals Inc Paragon Biotek, Inc. Instituted Final written decision All inst. claims upheld Innovative Environmental PGR2016-00002 9,126,245 19-Nov-15 Peroxychem LLC Technologies, Inc. Instituted Settled post-institution N/A Dr. Reddy’s Laboratories, PGR2016-00007 Aloxi® 9,173,942 5-Feb-16 Ltd. Helsinn Healthcare S.A. Denied N/A N/A Dr. Reddy’s Laboratories, PGR2016-00008 Aloxi® 9,173,942 5-Feb-16 Ltd. Helsinn Healthcare S.A. Denied N/A N/A Copaxone®, Yeda Research and Development PGR2016-00010 Glatopa® 9,155,776 16-Feb-16 Mylan N.V. Co Ltd. Denied Procedurally dismissed N/A PGR2016-00018 9,091,698 27-Apr-16 B.R.A.H.M.S. France S.A.S. Becton, Dickinson and Company Instituted Settled post-institution N/A Copaxone®, Yeda Research and Development PGR2016-00028 Glatopa® 9,155,776 12-Jul-16 Amneal Pharmaceuticals LLC Co Ltd. N/A Settled pre-institution N/A PGR2016-00042 9,415,105 12-Sep-16 Corvus Pharmaceuticals, Inc. The United States of America Denied Claims disclaimed N/A All inst.