Scott-Macon Healthcare Review: Fourth Quarter 2012
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Update from the CEO by Charles Jaffe, MD, Phd, HL7 CEO HL7’S 22Nd Annual Plenary Meeting
AUGUST 2008 In This Issue... Update from the CEO By Charles Jaffe, MD, PhD, HL7 CEO HL7’s 22nd Annual Plenary Meeting..... 2 Work Group Co-Chair Elections: As the languid days of August almost impercep- Development Organizations What to Expect in Vancouver................ 3 tibly transform into the cool autumn nights of (SDOs). Most importantly, September, some significant milestones emerged Update from Headquarters.............. 4-5 it is a living document. The from the HL7 landscape. Roadmap development team ExL Pharma’s 4th Annual EHR and will continue to be com- Charles Jaffe, MD, PhD eClinical Technologies Conference....... 6 The membership of HL7 has its very first posed of a broad constitu- News from the PMO.............................. 6 Roadmap. A strategic plan for technical and busi- ency with distinct requirements, and with plans to ness development is embodied in this document. It publish Roadmap updates annually. Version 3 Normative Edition 2008 Now Available...................................... 7 is more than just a promise to our stakeholders; the Roadmap provides the end-user community, gov- As the Roadmap matures, it will reflect the changing Multiple Modes of Delivering ernment agencies, software developers and other Education............................................. 8 business needs of our organization. More opportuni- standards development organizations clear guide- ties require more creative resource development and Advisory Council News....................... 9 lines for our products and services. In addition, the new means of expanding our funding model. Co-Chair Election Results.................... 9 Roadmap offers something beyond a commitment to develop a new standard or a new release of a Part of our new funding model emerged with the HL7 Certification Exam balloted one. -
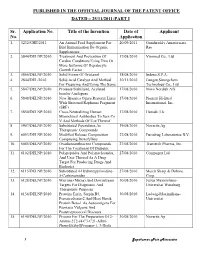
Published in the Official Journal of the Patent Office Dated – 25/11/2011-Part I
PUBLISHED IN THE OFFICIAL JOURNAL OF THE PATENT OFFICE DATED – 25/11/2011-PART I Sr. Application No. Title of the Invention Date of Applicant No. Application 1. 3232/CHE/2011 An Animal Feed Supplement For 20/09/2011 Gundareddy Amareswara Bird Immunization By Organic Rao Supplements 2. 5844/DELNP/2010 Treatment And Prevention Of 17/08/2010 Viromed Co., Ltd Cardiac Conditions Using Two Or More Isoforms Of Hepatocyte Growth Factor 3. 5866/DELNP/2010 Solid Forms Of Ortataxel 18/08/2010 Indena S.P.A. 4. 2844/DEL/2010 Solid Acid Catalyst And Method 30/11/2010 Jiangsu Sinorgchem For Preparing And Using The Same Technology Co., Ltd 5. 5847/DELNP/2010 Protease Stabilized, Acylated 17/08/2010 Novo Nordisk A/S Insulin Analogues 6. 5848/DELNP/2010 New Brassica Ogura Restorer Lines 17/08/2010 Pioneer Hi-Bred With Shotened Raphanus Fragment International, Inc. (SRF) 7. 5850/DELNP/2010 Cross-Neutralizing Human 17/08/2010 Humab, Llc Monoclonal Antibodies To Sars-Co V And Methods Of Use Thereof 8. 5907/DELNP/2010 Substituted Piperidines As 19/08/2010 Novartis Ag Therapeutic Compounds 9. 6093/DELNP/2010 Modified Release Composition 27/08/2010 Eurodrug Laboratories B.V. Comprising Doxofylline 10. 6085/DELNP/2010 Oxadiazoanthracene Compounds 27/08/2010 Transtech Pharma, Inc. For The Treatment Of Diabetes 11. 6102/DELNP/2010 Polypeptides And Polynucleotides, 27/08/2010 Compugen Ltd And Uses Thereof As A Drug Target For Producing Drugs And Biologics 12. 6115/DELNP/2010 Substituted 4-Hydroxypyrimidine- 27/08/2010 Merck Sharp & Dohme 5-Carboxamides Corp. 13. 6128/DELNP/2010 Microna (Mirna) And Downstream 30/08/2010 Julius Maximilians- Targets For Diagnostic And Universitat Wurzburg Therapeutic Purposes 14. -

HL7 Mobile Health Work Group
SEPTEMBER 2013 HL7 Mobile Health Work Group 101 By Gora Datta, Co-Chair, HL7 Mobile Health Work Group; Co-Lead, HL7 In- teroperability EHR Work Group; HL7 Ambassador; Group Chairman & CEO, CAL2CAL Corporation The HL7 Mobile Health (MH) Work Group is now • Identify and one year old! Over the past year, the work group promote mobile (WG) has rapidly matured from loosely held health concepts interested individuals to a cohesive group of ex- for interoper- perts that meets regularly. We welcome one and ability as Gora Datta all to join our Mobile Friday weekly calls every adopted and Friday at 11am ET. adapted for use in the mobile environment. • Coordinate and cooperate with other The group’s mission “The HL7 Mobile Health groups interested in using mobile health to Work Group creates and promotes health infor- promote health, wellness, public health, mation technology standards and frameworks for clinical, social media, and other settings. mobile health” captures the essence of its charter, • Provide a forum where HL7 members and as outlined: stakeholders collaborate in standardizing • Identify (and develop, as applicable) data to enable the secure exchange, storage, standards and functional requirements that analysis, and transmission of data and are specific to the mobile health environment. information for mobile applications and/ or mobile devices. One of the challenges the group has is that it is one of the few HL7 groups (like the EHR WG) that is not domain specific; and is more horizontal in nature. Therefore, its charter cuts across other vertical/domain oriented (work) groups. Given the tremendous interest and participation not only in the US but also globally, the HL7 Mobile Health WG is rapidly stepping up to identify and fill the gaps in the mobile health standards space. -

EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
December 22, 2008 EP Vantage Interview - Silence hoping to have something to shout about in 2009 Lisa Urquhart With its first product expected to go into the clinic potentially as early as January, Silence Therapeutics is hoping that 2009 will be year it has something to shout about and one that will reverse the alarming share price decline that has seen the company’s valuation slip from a high of over £170m in June 2007 to £21.6m today. Silence is one of a growing number of companies working in RNA interference (RNAi) and particularly short interfering RNA (siRNA), which work by selectively silencing or inactivating genes related to certain diseases. It is importantly one of only two companies that have composition of matter patent protection for their siRNA drugs. Speaking to EP Vantage, Iain Ross, chief executive of Silence, says: “It’s a big year coming up for us.” The group has spent most of the last 12 months strengthening its IP position, and next year comes the important move of the group’s lead candidate Atu027 into the clinic, an event Mr Ross says should take place in the first quarter of the year. Many expect it could be as soon as the end of January. Partnering focus What is less expected is the speed at which Mr Ross intends to partner the drug, which is being developed in solid tumours, with an emphasis on lung cancer. “We would be looking at doing something either at the end of 2009 or the beginning of 2010,” he says. Key to partnering discussions will be the drug demonstrating good safety data in a number of cancer patients, which could be the catalyst to starting talks with big pharma who have already started to ask about Atu027. -

9894 Pharma Tech Media Planner V6 2007
www.pharmtech.com years 1977– 2007 30ANNIVERSARY CELEBRATING 30 YEARS AS THE 2007 INDUSTRY’S MOST AUTHORITATIVE SOURCE Media Planner years 1977–2007 ANNIVERSARY years 1977–2007 ANNIVERSARY THE PHARMACEUTICAL TECHNOLOGY BRAND PUBLISHER’S STATEMENT Pharmaceutical Technology’s authoritative reputation and powerful brand recognition within the pharmaceutical/biopharmaceutical development & manufacturing marketplace will help you establish and maintain your own strong brand among pharma industry decision makers. A circulation of 38,667 BPA-qualified subscribers* and unmatched peer written and reviewed editorial make Pharmaceutical Technology an invaluable resource within top pharma companies, as well as small, specialty and biotech pharma companies spending billions each year on pharmaceutical development and manufacturing. Please celebrate with us as Pharmaceutical Technology marks its 30th Anniversary as the industry leader. —Michael Tracey, Publisher % 90 of readers rated Pharmaceutical Technology as important or very important to them as a professionalˆ EDITORIAL MISSION Pharmaceutical Technology publishes authoritative, reliable, and timely peer-reviewed research and expert analyses for scientists, engineers, technicians, and managers engaged in process development, manufacturing, formulation, analytical technology, packaging and regulatory compliance in the pharmaceutical and biotechnology industries. —Douglas McCormick, Editor in chief www.pharmtech.com *BPA June 2006 Statement ^2006 Readership Study Conducted by Advanstar Research -

Memorandum JUL 1 6 201D
Memorandum Subject Date Additional Quota Letters Received by July 15, 2010 (DFN: 630-08.2) JUL 1 6 201D To From Christine A. Sanncrud. Ph.D., Chief "Barbar• J.Illoockholdt, Chief Drug & Chemical Evaluation Section Regul• torn Section Office of Diverison Control Off cL rl Diversion Control On July 15. 2010, this section received your e-mail requesting a review of seventeen (17) quota applications from sixteen (16) registered manufacturers to determine if there arc any pending administrative/legal actions against these applicants and to advise ODE of the findings. ODOR conducted reviews (NADDIS, CSA, etc), as well as surveyed the responsible field offices for their input and recommendations. Provided below are the results and recommendations. QUOTA APPLICANTS wan NO ADVERSE OR DEROGATORY INFORMATIO N Novartis Consumer I lealth Lincoln (10345) (b)(4);(b)(7)(E) Baxter (10346) Generics Bidco li bda Vintage (10347) Noramco Delaware (10348) Pharmaceuticals International inc. (10349) Pharmedium (10351) Pharmedium (10352) Rhodes (10356) Bio-Pharm (10357) Patheon (10358) Patheon (10359) Watson (10361) B & B (10363) lospira. Inc. NC (10364) Epic Pharma (10366) Mallinckrodt I lobart (10367) Chemtos (10368) Vol. II Page 55 2 Per consultation with the field offices. DEA does not have sufficient grounds to limit, restrict. or deny quota requests from these registrants. Based on this information. ODG suggests that you proceed with the completion of the quota applications. If you have any questions pertaining to this information. please feel free to contact me (b)(6);(b)(7)( or SC C) (b)(6);(b)(7)(C) Vol. II Page 56 Memorandum Subject Date Additional Quota Letters Received as of July 19, 201() (DFN: 630-08.2) stir 2 8 2010,., To Fr ,./"./ Christine A. -

Seven Members Honored HL7 Honored Seven Members with the 14Th Annual W
JANUARY 2011 Upcoming WORKING GROUP MEETINGS 2010 Ed Hammond Volunteer of the Year Awards Seven members honored HL7 honored seven members with the 14th annual W. Edward Hammond, PhD Volunteer of the Year Award. Established in 1997, the award is named after Dr. Ed Hammond, one of HL7’s most active volunteers, founding member and past Board chair. The award recognizes individuals who January 9 – 14, 2011 May 15 – 20, 2011 have made significant contributions to HL7’s success. The 2010 recipients include: • Hugh Glover and Julie James, HL7 UK January Working Group • Stan Huff, MD, chief medical informatics officer, Intermountain Healthcare Working Group Meeting • Charlie Mead, MD, MSc, CTO, 3rd Millennium, Inc. Meeting • Mark Shafarman, principal, Shafarman Consulting Hilton in the Walt Disney World Resort • D. Mead Walker, Health Data and Interoperability, Inc. Cliftons Meeting and Training Center Lake Buena Vista, FL • Pat Van Dyke, director of information, security, privacy and EDI representing Delta Dental and the Amora Hotel Plans Association Sydney, Australia About the Volunteers: Hugh Glover and Julie James have been involved with HL7 since 2001. They received the award jointly as they are partners both personally and profession- ally. They have both actively contributed to HL7 for many years and have held leadership positions in the Pharmacy Work Group such as a co-chair or facili- tator. Their backgrounds—James as a pharmacist and Glover’s expertise in data modeling—complement each other and have brought valuable insight to HL7. Both have been involved with Version 3 development since its inception. James September 11 – 16, 2011 January 15 – 20, 2012 is also active in the Patient Safety Work Group. -

Pharmaceutical Research and Manufacturers of America – Phrma
The Short-Term and Long-Term Competitive Impact of Authorized Generics A Report for the Federal Trade Commission October 28, 2009 TABLE OF CONTENTS INTRODUCTION ........................................................................................................................... 1 DISCUSSION .................................................................................................................................. 3 I. LONG-TERM COMPETITIVE HARM IS UNLIKELY ................................................... 3 A. Unsupported Foreclosure Claims Have Been Made For Nearly Two Decades .................................................................................................................... 4 B. Marketplace Realities Undercut The Long-Tenn Foreclosure Theory .................... 7 1. Sales and Profitability Are Growing ........................................................... 7 2. Wall Street Valuations Are Growing ........................................................ 10 II. THE FTC PRICING ANALYSIS SHOWS $880 MILLION IN CONSUMER SAVINGS .......................................................................................................................... 14 III. CONSUMER SAVINGS SHOULD BE MEASURED USING WHOLESALE DATA AND WEIGHTED AVERAGE PRICES .............................................................. 17 A. The Wholesale Data Carries Far More Weight.. .................................................... 17 B. Average Drug Prices Should Be Volume-Weighted ............................................. 19 IV. -

Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Paten
Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patents Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent DrugsDrug Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under -

Recent Advances in Oligonucleotide Therapeutics in Oncology
International Journal of Molecular Sciences Review Recent Advances in Oligonucleotide Therapeutics in Oncology Haoyu Xiong 1, Rakesh N. Veedu 2,3 and Sarah D. Diermeier 1,* 1 Department of Biochemistry, University of Otago, Dunedin 9016, New Zealand; [email protected] 2 Centre for Molecular Medicine and Innovative Therapeutics, Murdoch University, Perth 6150, Australia; [email protected] 3 Perron Institute for Neurological and Translational Science, Perth 6009, Australia * Correspondence: [email protected] Abstract: Cancer is one of the leading causes of death worldwide. Conventional therapies, including surgery, radiation, and chemotherapy have achieved increased survival rates for many types of cancer over the past decades. However, cancer recurrence and/or metastasis to distant organs remain major challenges, resulting in a large, unmet clinical need. Oligonucleotide therapeutics, which include antisense oligonucleotides, small interfering RNAs, and aptamers, show promising clinical outcomes for disease indications such as Duchenne muscular dystrophy, familial amyloid neuropathies, and macular degeneration. While no approved oligonucleotide drug currently exists for any type of cancer, results obtained in preclinical studies and clinical trials are encouraging. Here, we provide an overview of recent developments in the field of oligonucleotide therapeutics in oncology, review current clinical trials, and discuss associated challenges. Keywords: antisense oligonucleotides; siRNA; aptamers; DNAzymes; cancers Citation: Xiong, H.; Veedu, R.N.; 1. Introduction Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in According to the Global Cancer Statistics 2018, there were more than 18 million new Oncology. Int. J. Mol. Sci. 2021, 22, cancer cases and 9.6 million deaths caused by cancer in 2018 [1]. -

Clinical Research Services
Clinical Research Services Advancing the future of medicine through research. Who We Are Experience Clinical Research Services is a department within Clinical Research Services has been involved CHI St. Alexius Health in Bismarck, ND. We are fully in clinical research since 1987. Our professional dedicated to providing research support services staff has more than 180 years of combined to physicians within our region and the valued experience in clinical trials. We are committed patients they serve. to providing outstanding service to ensure the success of every project. CHI St. Alexius Health is a tertiary care facility affiliated with PrimeCare, a health group which Our involvement in inpatient and outpatient combines the most experienced, trusted, and phase II-IV clinical and device research studies has proven medical leaders in the area. PrimeCare is contributed substantially to the approval of new comprised of a network which includes more than drugs and treatments. You should carefully consider 190 physicians in private and institutional practice, both the benefits and the risks of participation including: CHI St. Alexius Health, Mid Dakota Clinic, before enrolling in a study. The Bone & Joint Center, and other affiliated area physicians. Many of these physicians are actively Previous clinical study trials: involved as investigators for clinical trials. • Oncology • Orthopaedic • Rheumatology • Diabetes Our clinical research team is committed to providing: • Neurology • Weight loss • Rapid study start-up • Cardiology • Pain • Pro-active patient enrollment • Urology • Men’s Health • Clean data submission • Gastroenterology • Women’s Health • Highly experienced principal • Infectious Disease • Medical Devices investigators • Pediatric • Initial training and continuing education for all support personnel Sponsors Patient Demographics Abbott Laboratories Characteristics of our patients include Acorda Therapeutics, Inc. -

יומן הפטנטים והמדגמים Patents and Designs Journal
י /' התשס"ח 5/2008 רשומות ISRAEL STATE RECORDS ו' באב התשס"ח August 7, 2008 יומן הפטנטים והמדגמים PATENTS AND DESIGNS JOURNAL פטנטים עמוד PATENTS Page בקשות שהוגשו Applications filed 1507 בקשות שקובלו Applications accepted 1775 פטנטים שניתנו Patents granted 1992 פטנטים שחודשו Patents renewed 1993 פטנטים שתוקפם פקעו Patents not in force 1995 פטנטים שחודשו לעשרים שנה Patents renewed for 20 years 1996 פטנטים שפג תוקפם Patents expired 1997 הודעות Notices 1998 שינויים בפרטים רשומים Changes in particulars entered בפנקס in register 2001 תיקוני טעויות Corrigenda 2002 מפתחות לבקשות שקובלו Indices of applications accepted i מדגמים DESIGNS מדגמים שנרשמו Designs registered 2004 מדגמים שחודשו Designs renewed 2017 מדגמים שבוטלו Designs void 2018 ו' באב התשס"ח – August 7, 2008 1507 ידיעות כלליות מכתבים, מסמכים, וכו' בענייני פטנטים ומדגמים יש לשלוח אל: רשם הפטנטים והמדגמים, רח' הסדנא 4, ירושלים לשכת הפטנטים נמצאת ברח' הסדנא 4, תלפיות, ירושלים והיא פתוחה לציבור בימי חול שאינם ערבי שבת או מועד בין השעות 8:30 ו - 12:30. לשכת הפטנטים מספקת תצלומים של פירוטים ושרטוטים במחיר של 2.50 שקלים בעד כל עמוד או חלק ממנו. אגרות ללשכת הפטנטים מתקבלות אך ורק על ידי תשלום לחשבון הלשכה בבנק הדואר מס' 0-24145-2. יש להציג קבלת בנק הדואר ללשכה יחד עם הבקשה לפעולה שעבורה האגרה שולמה. GENERAL INFORMATION Letters, documents, etc. concerning Patents and Designs should be addressed to: The Commissioner of Patents and Designs, 4 Hasadnah St., Jerusalem The Patent Office is located at 4 Hasadnah St., Talpiot, Jerusalem and is open to the public on weekdays, except on Fridays or on the eves of holydays, from 08:30 to 12:30 hrs.