Gene Therapy: Yesterday, Today and Tomorrow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Marginable OTC Stocks
List of Marginable OTC Stocks @ENTERTAINMENT, INC. ABACAN RESOURCE CORPORATION ACE CASH EXPRESS, INC. $.01 par common No par common $.01 par common 1ST BANCORP (Indiana) ABACUS DIRECT CORPORATION ACE*COMM CORPORATION $1.00 par common $.001 par common $.01 par common 1ST BERGEN BANCORP ABAXIS, INC. ACETO CORPORATION No par common No par common $.01 par common 1ST SOURCE CORPORATION ABC BANCORP (Georgia) ACMAT CORPORATION $1.00 par common $1.00 par common Class A, no par common Fixed rate cumulative trust preferred securities of 1st Source Capital ABC DISPENSING TECHNOLOGIES, INC. ACORN PRODUCTS, INC. Floating rate cumulative trust preferred $.01 par common $.001 par common securities of 1st Source ABC RAIL PRODUCTS CORPORATION ACRES GAMING INCORPORATED 3-D GEOPHYSICAL, INC. $.01 par common $.01 par common $.01 par common ABER RESOURCES LTD. ACRODYNE COMMUNICATIONS, INC. 3-D SYSTEMS CORPORATION No par common $.01 par common $.001 par common ABIGAIL ADAMS NATIONAL BANCORP, INC. †ACSYS, INC. 3COM CORPORATION $.01 par common No par common No par common ABINGTON BANCORP, INC. (Massachusetts) ACT MANUFACTURING, INC. 3D LABS INC. LIMITED $.10 par common $.01 par common $.01 par common ABIOMED, INC. ACT NETWORKS, INC. 3DFX INTERACTIVE, INC. $.01 par common $.01 par common No par common ABLE TELCOM HOLDING CORPORATION ACT TELECONFERENCING, INC. 3DO COMPANY, THE $.001 par common No par common $.01 par common ABR INFORMATION SERVICES INC. ACTEL CORPORATION 3DX TECHNOLOGIES, INC. $.01 par common $.001 par common $.01 par common ABRAMS INDUSTRIES, INC. ACTION PERFORMANCE COMPANIES, INC. 4 KIDS ENTERTAINMENT, INC. $1.00 par common $.01 par common $.01 par common 4FRONT TECHNOLOGIES, INC. -
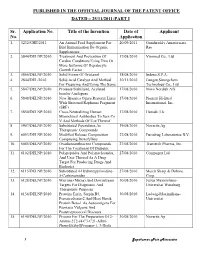
Published in the Official Journal of the Patent Office Dated – 25/11/2011-Part I
PUBLISHED IN THE OFFICIAL JOURNAL OF THE PATENT OFFICE DATED – 25/11/2011-PART I Sr. Application No. Title of the Invention Date of Applicant No. Application 1. 3232/CHE/2011 An Animal Feed Supplement For 20/09/2011 Gundareddy Amareswara Bird Immunization By Organic Rao Supplements 2. 5844/DELNP/2010 Treatment And Prevention Of 17/08/2010 Viromed Co., Ltd Cardiac Conditions Using Two Or More Isoforms Of Hepatocyte Growth Factor 3. 5866/DELNP/2010 Solid Forms Of Ortataxel 18/08/2010 Indena S.P.A. 4. 2844/DEL/2010 Solid Acid Catalyst And Method 30/11/2010 Jiangsu Sinorgchem For Preparing And Using The Same Technology Co., Ltd 5. 5847/DELNP/2010 Protease Stabilized, Acylated 17/08/2010 Novo Nordisk A/S Insulin Analogues 6. 5848/DELNP/2010 New Brassica Ogura Restorer Lines 17/08/2010 Pioneer Hi-Bred With Shotened Raphanus Fragment International, Inc. (SRF) 7. 5850/DELNP/2010 Cross-Neutralizing Human 17/08/2010 Humab, Llc Monoclonal Antibodies To Sars-Co V And Methods Of Use Thereof 8. 5907/DELNP/2010 Substituted Piperidines As 19/08/2010 Novartis Ag Therapeutic Compounds 9. 6093/DELNP/2010 Modified Release Composition 27/08/2010 Eurodrug Laboratories B.V. Comprising Doxofylline 10. 6085/DELNP/2010 Oxadiazoanthracene Compounds 27/08/2010 Transtech Pharma, Inc. For The Treatment Of Diabetes 11. 6102/DELNP/2010 Polypeptides And Polynucleotides, 27/08/2010 Compugen Ltd And Uses Thereof As A Drug Target For Producing Drugs And Biologics 12. 6115/DELNP/2010 Substituted 4-Hydroxypyrimidine- 27/08/2010 Merck Sharp & Dohme 5-Carboxamides Corp. 13. 6128/DELNP/2010 Microna (Mirna) And Downstream 30/08/2010 Julius Maximilians- Targets For Diagnostic And Universitat Wurzburg Therapeutic Purposes 14. -

EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
December 22, 2008 EP Vantage Interview - Silence hoping to have something to shout about in 2009 Lisa Urquhart With its first product expected to go into the clinic potentially as early as January, Silence Therapeutics is hoping that 2009 will be year it has something to shout about and one that will reverse the alarming share price decline that has seen the company’s valuation slip from a high of over £170m in June 2007 to £21.6m today. Silence is one of a growing number of companies working in RNA interference (RNAi) and particularly short interfering RNA (siRNA), which work by selectively silencing or inactivating genes related to certain diseases. It is importantly one of only two companies that have composition of matter patent protection for their siRNA drugs. Speaking to EP Vantage, Iain Ross, chief executive of Silence, says: “It’s a big year coming up for us.” The group has spent most of the last 12 months strengthening its IP position, and next year comes the important move of the group’s lead candidate Atu027 into the clinic, an event Mr Ross says should take place in the first quarter of the year. Many expect it could be as soon as the end of January. Partnering focus What is less expected is the speed at which Mr Ross intends to partner the drug, which is being developed in solid tumours, with an emphasis on lung cancer. “We would be looking at doing something either at the end of 2009 or the beginning of 2010,” he says. Key to partnering discussions will be the drug demonstrating good safety data in a number of cancer patients, which could be the catalyst to starting talks with big pharma who have already started to ask about Atu027. -

Guidelines with Regard to the Composition, Calculation and Management of the Index
INDEX METHODOLOGY Solactive Pharma Breakthrough Value Index Version 2.1 dated September 03, 2020 Contents Important Information 1. Index specifications 1.1 Short Name and ISIN 1.2 Initial Value 1.3 Distribution 1.4 Prices and Calculation Frequency 1.5 Weighting 1.6 Index Committee 1.7 Publication 1.8 Historical Data 1.9 Licensing 2. Composition of the Index 2.1 Selection of the Index Components 2.2 Ordinary Adjustment 2.3 Extraordinary Adjustment 3. Calculation of the Index 3.1 Index Formula 3.2 Accuracy 3.3 Adjustments 3.4 Dividends and other Distributions 3.5 Corporate Actions 3.6 Correction Policy 3.7 Market Disruption 3.8 Consequences of an Extraordinary Event 4. Definitions 5. Appendix 5.1 Contact Details 5.2 Calculation of the Index – Change in Calculation Method 2 Important Information This document (“Index Methodology Document”) contains the underlying principles and regulations regarding the structure and the operating of the Solactive Pharma Breakthrough Value Index. Solactive AG shall make every effort to implement regulations. Solactive AG does not offer any explicit or tacit guarantee or assurance, neither pertaining to the results from the use of the Index nor the Index value at any certain point in time nor in any other respect. The Index is merely calculated and published by Solactive AG and it strives to the best of its ability to ensure the correctness of the calculation. There is no obligation for Solactive AG – irrespective of possible obligations to issuers – to advise third parties, including investors and/or financial intermediaries, of any errors in the Index. -

Healthcare & Life Sciences Industry Update
Healthcare & Life Sciences Industry Update August 2011 Member FINRA/SIPC www.harriswilliams.com What We’ve Been Reading August 2011 • The biggest story from inside the Beltway over the last month was the battle and eventual deal to raise the U.S. debt ceiling to avoid an impending August 2nd default. The Budget Control Act of 2011 will raise the debt ceiling by $2.1-$2.4 trillion, while reducing spending by approximately $2.1 trillion over the next decade. In an interesting post-deal analysis, Michael Hiltzik of the Los Angeles Times notes that despite the protracted negotiations over a broad spectrum of cost-cutting measures, healthcare was not raised as a primary issue. Some have found this especially concerning, considering the outsized portion of the Federal Budget currently allocated to healthcare spending, which is forecast to rise in the coming years. A recent article in The Economist, entitled “Looking to Uncle Sam” makes this point and goes further to suggest that Centers for Medicare & Medicaid Services (CMS) actuaries may be underestimating future increases in Medicare and Medicaid spending. • One potential reason CMS actuaries may be underestimating the future costs of Medicare and Medicaid relates to healthcare reform’s effect on employee benefits, and specifically, the 2014 switch to subsidized exchange policies. A recent report by McKinsey & Company estimates that approximately 30% of employers will stop offering employer- sponsored insurance (ESI) when this switch is made, as opposed to the 7% estimated by the Congressional Budget Office. An ESI exodus of this magnitude could cause substantial strain on an already robust government healthcare budget. -

Recent Advances in Oligonucleotide Therapeutics in Oncology
International Journal of Molecular Sciences Review Recent Advances in Oligonucleotide Therapeutics in Oncology Haoyu Xiong 1, Rakesh N. Veedu 2,3 and Sarah D. Diermeier 1,* 1 Department of Biochemistry, University of Otago, Dunedin 9016, New Zealand; [email protected] 2 Centre for Molecular Medicine and Innovative Therapeutics, Murdoch University, Perth 6150, Australia; [email protected] 3 Perron Institute for Neurological and Translational Science, Perth 6009, Australia * Correspondence: [email protected] Abstract: Cancer is one of the leading causes of death worldwide. Conventional therapies, including surgery, radiation, and chemotherapy have achieved increased survival rates for many types of cancer over the past decades. However, cancer recurrence and/or metastasis to distant organs remain major challenges, resulting in a large, unmet clinical need. Oligonucleotide therapeutics, which include antisense oligonucleotides, small interfering RNAs, and aptamers, show promising clinical outcomes for disease indications such as Duchenne muscular dystrophy, familial amyloid neuropathies, and macular degeneration. While no approved oligonucleotide drug currently exists for any type of cancer, results obtained in preclinical studies and clinical trials are encouraging. Here, we provide an overview of recent developments in the field of oligonucleotide therapeutics in oncology, review current clinical trials, and discuss associated challenges. Keywords: antisense oligonucleotides; siRNA; aptamers; DNAzymes; cancers Citation: Xiong, H.; Veedu, R.N.; 1. Introduction Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in According to the Global Cancer Statistics 2018, there were more than 18 million new Oncology. Int. J. Mol. Sci. 2021, 22, cancer cases and 9.6 million deaths caused by cancer in 2018 [1]. -

2016 Medicines in Development for Rare Diseases a LIST of ORPHAN DRUGS in the PIPELINE
2016 Medicines in Development for Rare Diseases A LIST OF ORPHAN DRUGS IN THE PIPELINE Autoimmune Diseases Product Name Sponsor Official FDA Designation* Development Status Actemra® Genentech treatment of systemic sclerosis Phase III tocilizumab South San Francisco, CA www.gene.com Adempas® Bayer HealthCare Pharmaceuticals treatment of systemic sclerosis Phase II riociguat Whippany, NJ www.pharma.bayer.com ARA 290 Araim Pharmaceuticals treatment of neuropathic pain in patients Phase II Tarrytown, NY with sarcoidosis www.ariampharma.com ARG201 arGentis Pharmaceuticals treatment of diffuse systemic sclerosis Phase II (type 1 native bovine skin Collierville, TN www.argentisrx.com collagen) BYM338 Novartis Pharmaceuticals treatment of inclusion body myositis Phase III (bimagrumab) East Hanover, NJ www.novartis.com CCX168 ChemoCentryx treatment of anti-neutrophil cytoplasmic Phase II (5a receptor antagonist) Mountain View, CA auto-antibodies associated vasculitides www.chemocentryx.com (granulomatosis with polyangitis or Wegener's granulomatosis), microscopic polyangitis, and Churg-Strauss syndrome * This designation is issued by the FDA's Office of Orphan Products Development while the drug is still in development. The designation makes the sponsor of the drug eligible for entitlements under the Orphan Drug Act of 1983. The entitlements include seven years of marketing exclusivity following FDA approval of the drug for the designated use. Medicines in Development: Rare Diseases | 2016 1 Autoimmune Diseases Product Name Sponsor Official FDA -

יומן הפטנטים והמדגמים Patents and Designs Journal
י /' התשס"ח 5/2008 רשומות ISRAEL STATE RECORDS ו' באב התשס"ח August 7, 2008 יומן הפטנטים והמדגמים PATENTS AND DESIGNS JOURNAL פטנטים עמוד PATENTS Page בקשות שהוגשו Applications filed 1507 בקשות שקובלו Applications accepted 1775 פטנטים שניתנו Patents granted 1992 פטנטים שחודשו Patents renewed 1993 פטנטים שתוקפם פקעו Patents not in force 1995 פטנטים שחודשו לעשרים שנה Patents renewed for 20 years 1996 פטנטים שפג תוקפם Patents expired 1997 הודעות Notices 1998 שינויים בפרטים רשומים Changes in particulars entered בפנקס in register 2001 תיקוני טעויות Corrigenda 2002 מפתחות לבקשות שקובלו Indices of applications accepted i מדגמים DESIGNS מדגמים שנרשמו Designs registered 2004 מדגמים שחודשו Designs renewed 2017 מדגמים שבוטלו Designs void 2018 ו' באב התשס"ח – August 7, 2008 1507 ידיעות כלליות מכתבים, מסמכים, וכו' בענייני פטנטים ומדגמים יש לשלוח אל: רשם הפטנטים והמדגמים, רח' הסדנא 4, ירושלים לשכת הפטנטים נמצאת ברח' הסדנא 4, תלפיות, ירושלים והיא פתוחה לציבור בימי חול שאינם ערבי שבת או מועד בין השעות 8:30 ו - 12:30. לשכת הפטנטים מספקת תצלומים של פירוטים ושרטוטים במחיר של 2.50 שקלים בעד כל עמוד או חלק ממנו. אגרות ללשכת הפטנטים מתקבלות אך ורק על ידי תשלום לחשבון הלשכה בבנק הדואר מס' 0-24145-2. יש להציג קבלת בנק הדואר ללשכה יחד עם הבקשה לפעולה שעבורה האגרה שולמה. GENERAL INFORMATION Letters, documents, etc. concerning Patents and Designs should be addressed to: The Commissioner of Patents and Designs, 4 Hasadnah St., Jerusalem The Patent Office is located at 4 Hasadnah St., Talpiot, Jerusalem and is open to the public on weekdays, except on Fridays or on the eves of holydays, from 08:30 to 12:30 hrs. -

Combining High-Sensitivity Troponin with the AHA/ACC Cholesterol
Combining High -Sensitivity Troponin with the AHA/ACC Cholesterol Guidelines To Guide Evolocumab Therapy Nicholas A. Marston, MD, MPH, Kazuma Oyama, MD, PhD, Petr Jarolim, MD, PhD, Minao Tang, MS, Peter S. Sever, PhD, FRCP, Anthony C. Keech, MD, Armando Lira Pineda, MD, Huei Wang, PhD, Robert P. Giugliano, MD, SM, Marc S. Sabatine, MD, MPH, David A. Morrow, MD, MPH Insights into Plaque Vulnerability, Stabilization and Mechanisms of Death in Stable Coronary Artery Disease Friday, November 13th, 2020 #AHA20 Disclosures Presenter: clinical trial involvement with Amgen, Pfizer, Novartis, and AstraZeneca without personal fees, payments, or salary increase. Co-Authors: KO reports grant support from JSPS Overseas Research Fellowships. PJ reports research support from Abbott Laboratories, Amgen, Inc., AstraZeneca, LP, Daiichi-Sankyo, Inc., Eisai, Inc., GlaxoSmithKline, Merck & Co., Inc., Regeneron Pharmaceuticals, Inc., Roche Diagnostics Corporation, Siemens Healthineers, Takeda Global Research and Development Center, and Waters Technologies Corporation, and consulting fees from Roche Diagnostics Corporation. MT reports no disclosures. PS reports research grants and honoraria for speakers bureau- Amgen and Pfizer. ACK reports grants and personal fees from Abbott, personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Mylan, personal fees from Pfizer, grants from Sanofi, grants from Novartis, personal fees from Bayer, outside the submitted work. ALP was previously employed by Amgen, now employed by Arrowhead Pharmaceutical. -

ACC.17 Program Committee Disclosures.Xlsx
ACC.17 Program Committee Disclosures of Relationships With Industry Name Disclosures Brian G. Abbott, MD, FACC Nothing to Disclose J. Dawn Abbott, MD, FACC DATA SAFETY MONITORING BOARD - Pfizer Nazem Akoum, MD, FACC CONSULTANT FEES/HONORARIA - Biotronik Karen P. Alexander, MD, FACC CONSULTANT FEES/HONORARIA - Gilead Sciences, Inc.; DATA SAFETY MONITORING BOARD - CytRx; RESEARCH/RESEARCH GRANTS - gilead, Regeneron, Sanofi Aventis Mouaz H. Al-Mallah, MD, FACC CONSULTANT FEES/HONORARIA - GE Healthcare; SPEAKER’S BUREAU - Pfizer Inc, Philips Jesus G. Almendral, MD, FACC Nothing to Disclose Aimee K. Armstrong, MD, FACC CONSULTANT FEES/HONORARIA - B. Braun Interventional Systems Inc., Edwards Lifesciences, Siemens Healthcare AX, St. Jude Medical; RESEARCH/RESEARCH GRANTS - Edwards Lifesciences, MEDTRONIC Herbert D. Aronow, MD, MPH, Nothing to Disclose FACC Samuel J. Asirvatham, MBBS, CONSULTANT FEES/HONORARIA - Abiomed, AtriCure, Biosense Webster, Biotronik, FACC Boston Scientific, Elsevier, Medtelligence, MEDTRONIC, Sanofi Aventis, St. Jude Medical, Wolters Kluwer, Zoll Alison Bailey, MD, FACC RESEARCH/RESEARCH GRANTS - CSL Behring Subhash Banerjee, MD, FACC CONSULTANT FEES/HONORARIA - Boston Scientific; OWNERSHIP INTEREST/PARTNERSHIP/PRINCIPAL - Mdcare Global LLC; RESEARCH/RESEARCH GRANTS - Boston Scientific Corporation; SPEAKER’S BUREAU - CSI, MEDTRONIC Geoffrey D. Barnes, MD, FACC CONSULTANT FEES/HONORARIA - Portola; RESEARCH/RESEARCH GRANTS - Blue Cross Blue Shield of Michigan, BMS/Pfizer Theodore A. Bass, MD, FACC Nothing to Disclose -

Medical Research Council
Elan Pharmaceuticals University of Kansas Parke-Davis Basilea Pharmaceutica Office of Rare Diseases (ORD) Swedish Orphan Biovitrum Astellas Pharma US, Inc. Food and Drug Administration (FDA) Biogen Idec AVEO Pharmaceuticals, Inc. Bioclin Research Laboratories Rare Diseases Clinical Research Network Birmingham Clinical Trials Unit Astellas Pharma Inc Baylor College of Medicine Astellas Pharma Europe BV Abbott Biotherapeutics Corp. OSI Pharmaceuticals Anglo-European College of Chiropractic The Beverage Institute. National Development and Research Institutes, Inc. Boston University University of Rochester Medstar Research Institute Omnicare Clinical Research ABX-CRO Bio-Kinetic Europe Ltd MRC Human Nutrition Research, Cambridge, UK. Shionogi Astellas Pharma Global Development, Inc. Ethicon, Inc. Symphogen A/S Averion-Hesperion Quintiles Laboratories Temple University Emory University National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) European Chiropractors Union Children's Hospital Boston Colchester Hospital University NHS Foundation Trust Covance GE Healthcare EMSI National Institute for Chiropractic Research Muscular Dystrophy Association Phoenix Children's Hospital OMRIX Biopharmaceuticals University of California, Los Angeles Cancer and Leukemia Group B University Health Network, Toronto University of Iowa Sarcoma Alliance for Research through Collaboration Westat Medivation, Inc. International Breast Cancer Study Group National Institute of Neurological Disorders and Stroke (NINDS) ClinPhone, Inc. Southwest Oncology Group Multi-Imagem and CDPI, Rio de Janeiro, Brasil Dutch Cancer Society Gary Cutter, PhD Murdoch Childrens Research Institute Chiltern International Inc. Janssen Research & Development, LLC Birmingham Children's Hospital NHS Foundation Trust Southwestern Oncology Group (SWOG) CIHR Canadian HIV Trials Network Royal Liverpool University Hospital Vertex Pharmaceuticals Incorporated UCB, Inc. Royal Hospital for Sick Children Trimeris Cystic Fibrosis Foundation Eisai Limited Eisai Inc. -

Safety and Activity of Autologous T Cells with Enhanced
D’Angelo SP1, Blay J-Y2, Chow W3, Demetri G4, Thistlethwaite F5, Sen S6, Razak A7, Safety and Activity of Autologous T Cells With Enhanced NY-ESO-1–Specific T-Cell Receptor Haanen J8, Noujaim J9, Johnson ML10, Laetsch TW11, Chiou VL12, Pearce L12, Faitg TH12, Ji R12, Johnson LA12, Shalabi A12, Thornton K4, Mackall C13, Van Tine BA14 (GSK3377794) in HLA-A*02+ Previously-Treated and -Untreated Patients With Advanced 1Memorial Sloan Kettering Cancer Center, New York, NY, USA; 2Département de Cancérologie Médicale, Centre Léon Bérard, Lyon, France; 3City of Hope Comprehensive Cancer Center, Duarte, CA, USA; 4Dana-Farber Cancer Institute and Ludwig Center at Harvard Medical School, Boston, MA, USA; 5The Christie NHS Foundation Trust and University of Manchester, Manchester, UK; 6Sarah Cannon Research Metastatic/Unresectable Synovial Sarcoma: A Master Protocol Study Design (IGNYTE-ESO) Institute, Denver, CO, USA; 7Princess Margaret Cancer Centre and Mount Sinai Hospital, Toronto, ON, Canada; 8Plesmanlaan 121, Antoni van Leeuwenhoek Ziekenhuis, Amsterdam, Netherlands; 9Institut D’Hématologie-Oncologie, Hôpital Maisonneuve-Rosemont, Montreal, Poster No. 459 QC, Canada; 10Sarah Cannon Research Institute, Nashville, TN, USA; 11University of Texas Southwestern Medical Center, Dallas, TX, USA; 12GlaxoSmithKline, Philadelphia, PA, USA; 13Stanford University, Palo Alto, CA, USA; 14Washington University in St. Louis, St. Louis, MO, USA Background Study design Unmet need This trial (IGNYTE-ESO; NCT03967223) has a Master Protocol design consisting of