RIN2 Deficiency Results in Macrocephaly, Alopecia, Cutis Laxa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pfeiffer Syndrome Type II Discovered Perinatally
Diagnostic and Interventional Imaging (2012) 93, 785—789 CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector LETTER / Musculoskeletal imaging Pfeiffer syndrome type II discovered perinatally: Report of an observation and review of the literature a,∗ a a a H. Ben Hamouda , Y. Tlili , S. Ghanmi , H. Soua , b c b a S. Jerbi , M.M. Souissi , H. Hamza , M.T. Sfar a Unité de néonatologie, service de pédiatrie, CHU Tahar Sfar, 5111 Mahdia, Tunisia b Service de radiologie, CHU Tahar Sfar, 5111 Mahdia, Tunisia c Service de gynéco-obstétrique, CHU Tahar Sfar, 5111 Mahdia, Tunisia Pfeiffer syndrome, described for the first time by Pfeiffer in 1964, is a rare hereditary KEYWORDS condition combining osteochondrodysplasia with craniosynostosis [1]. This syndrome is Pfeiffer syndrome; also called acrocephalosyndactyly type 5, which is divided into three sub-types. Type I Cloverleaf skull; is the classic Pfeiffer syndrome, with autosomal dominant transmission, often associated Craniosynostosis; with normal intelligence. Types II and III occur as sporadic cases in individuals who have Syndactyly; craniosynostosis with broad thumbs, broad big toes, ankylosis of the elbows and visceral Prenatal diagnosis abnormalities [2]. We report a case of Pfeiffer syndrome type II, discovered perinatally, which is distinguished from type III by the skull appearing like a cloverleaf, and we shall discuss the clinical, radiological and evolutive features and the advantage of prenatal diagnosis of this syndrome with a review of the literature. Observation The case involved a male premature baby born at 36 weeks of amenorrhoea with multiple deformities at birth. The parents were not blood-related and in good health who had two other boys and a girl with normal morphology. -

Lordosis, Kyphosis, and Scoliosis
SPINAL CURVATURES: LORDOSIS, KYPHOSIS, AND SCOLIOSIS The human spine normally curves to aid in stability or balance and to assist in absorbing shock during movement. These gentle curves can be seen from the side or lateral view of the spine. When viewed from the back, the spine should run straight down the middle of the back. When there are abnormalities or changes in the natural spinal curvature, these abnormalities are named with the following conditions and include the following symptoms. LORDOSIS Some lordosis is normal in the lower portion or, lumbar section, of the human spine. A decreased or exaggerated amount of lordosis that is causing spinal instability is a condition that may affect some patients. Symptoms of Lordosis include: ● Appearance of sway back where the lower back region has a pronounced curve and looks hollow with a pronounced buttock area ● Difficulty with movement in certain directions ● Low back pain KYPHOSIS This condition is diagnosed when the patient has a rounded upper back and the spine is bent over or curved more than 50 degrees. Symptoms of Kyphosis include: ● Curved or hunched upper back ● Patient’s head that leans forward ● May have upper back pain ● Experiences upper back discomfort after movement or exercise SCOLIOSIS The most common of the three curvatures. This condition is diagnosed when the spine looks like a “s” or “c” from the back. The spine is not straight up and down but has a curve or two running side-to-side. Sagittal Balance Definition • Sagittal= front-to-back direction (sagittal plane) • Imbalance= Lack of harmony or balance Etiology • Excessive lordosis (backwards lean) or kyphosis (forward lean) • Traumatic injury • Previous spinal fusion that disrupted sagittal balance Effects • Low back pain • Difficulty walking • Inability to look straight ahead when upright The most ergonomic and natural posture is to maintain neutral balance, with the head positioned over the shoulders and pelvis. -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

SCOLIOSIS: What Is New and True? Natural History, Screening/Evaluation and Treatment
SCOLIOSIS: what is new and true? Natural history, screening/evaluation and treatment Kathleen Moen MD Swedish Pediatric Specialty Update January 24, 2020 OBJECTIVES • 1) review of entity adolescent idiopathic scoliosis • 2) review the natural history of treated and untreated disease • 3) review screening recommendations and delineating patients who warrant referral • 4) review of treatment modalities Scoliosis • Definition: a lateral spinal curvature measuring at least 10 degrees on xray • Complex 3dimensional spinal deformity Adolescent Idiopathic Scoliosis • AIS: Scoliosis most often develops and progresses during the most rapid times of growth, typically between ages 10 ‐15 years. • NOT infantile or juvenile scoliosis which have different natural history profiles • NOT congenital spinal deformities, neuromuscular scoliosis or spinal dysraphism Adolescent Idiopathic Scoliosis • Classic Teaching – Small curves are common – Progression occurs during most rapid times of growth – Female predominance – Big curves get bigger – Etiology unclear: familial predisposition, and? – Idiopathic scoliosis not typically painful Adolescent Idiopathic Scoliosis • Mild scoliosis curves are common, affecting 2‐3% of the adolescent population, males and females equally • Less than 0.1% of adolescents have curves greater than 40 degrees. • Ratio of females to males 10:1 Cobb Angle Female:Male Prevalence >10 1.4‐2 :1 2 ‐ 3 % >20 5:1 .3 ‐ .5 % >30 10:1 .1 ‐ .3 % >40 10:1 <0.1% Weinstein, SL Adolescent Idiopathic Scoliosis, Prevalence and Natural History. Instructional -

Genetics of Congenital Hand Anomalies
G. C. Schwabe1 S. Mundlos2 Genetics of Congenital Hand Anomalies Die Genetik angeborener Handfehlbildungen Original Article Abstract Zusammenfassung Congenital limb malformations exhibit a wide spectrum of phe- Angeborene Handfehlbildungen sind durch ein breites Spektrum notypic manifestations and may occur as an isolated malforma- an phänotypischen Manifestationen gekennzeichnet. Sie treten tion and as part of a syndrome. They are individually rare, but als isolierte Malformation oder als Teil verschiedener Syndrome due to their overall frequency and severity they are of clinical auf. Die einzelnen Formen kongenitaler Handfehlbildungen sind relevance. In recent years, increasing knowledge of the molecu- selten, besitzen aber aufgrund ihrer Häufigkeit insgesamt und lar basis of embryonic development has significantly enhanced der hohen Belastung für Betroffene erhebliche klinische Rele- our understanding of congenital limb malformations. In addi- vanz. Die fortschreitende Erkenntnis über die molekularen Me- tion, genetic studies have revealed the molecular basis of an in- chanismen der Embryonalentwicklung haben in den letzten Jah- creasing number of conditions with primary or secondary limb ren wesentlich dazu beigetragen, die genetischen Ursachen kon- involvement. The molecular findings have led to a regrouping of genitaler Malformationen besser zu verstehen. Der hohe Grad an malformations in genetic terms. However, the establishment of phänotypischer Variabilität kongenitaler Handfehlbildungen er- precise genotype-phenotype correlations for limb malforma- schwert jedoch eine Etablierung präziser Genotyp-Phänotyp- tions is difficult due to the high degree of phenotypic variability. Korrelationen. In diesem Übersichtsartikel präsentieren wir das We present an overview of congenital limb malformations based Spektrum kongenitaler Malformationen, basierend auf einer ent- 85 on an anatomic and genetic concept reflecting recent molecular wicklungsbiologischen, anatomischen und genetischen Klassifi- and developmental insights. -

Macrocephaly Information Sheet 6-13-19
Next Generation Sequencing Panel for Macrocephaly Clinical Features: Macrocephaly refers to an abnormally large head, OFC greater than 98th percentile, inclusive of the scalp, cranial bone and intracranial contents. Megalencephaly, brain weight/volume ratio greater than 98th percentile, results from true enlargement of the brain parenchyma [1]. Megalencephaly is typically accompanied by macrocephaly, however macrocephaly can occur in the absence of megalencephaly [2]. Both macrocephaly and megalencephaly can been seen as isolated clinical findings as well as clinical features of a mutli-systemic syndromic diagnosis. Our Macrocephaly Panel includes analysis of the 36 genes listed below. Macrocephaly Sequencing Panel ASXL2 GLI3 MTOR PPP2R5D TCF20 BRWD3 GPC3 NFIA PTEN TBC1D7 CHD4 HEPACAM NFIX RAB39B UPF3B CHD8 HERC1 NONO RIN2 ZBTB20 CUL4B KPTN NSD1 RNF125 DNMT3A MED12 OFD1 RNF135 EED MITF PIGA SEC23B EZH2 MLC1 PPP1CB SETD2 Gene Clinical Features Details ASXL2 Shashi-Pena Shashi et al. (2016) found that six patients with developmental delay, syndrome macrocephaly, and dysmorphic features were found to have de novo truncating variants in ASXL2 [3]. Distinguishing features were macrocephaly, absence of growth retardation, and variability in the degree of intellectual disabilities The phenotype also consisted of prominent eyes, arched eyebrows, hypertelorism, a glabellar nevus flammeus, neonatal feeding difficulties and hypotonia. BRWD3 X-linked intellectual Truncating mutations in the BRWD3 gene have been described in males with disability nonsyndromic intellectual disability and macrocephaly [4]. Other features include a prominent forehead and large cupped ears. CHD4 Sifrim-Hitz-Weiss Weiss et al., 2016, identified five individuals with de novo missense variants in the syndrome CHD4 gene with intellectual disabilities and distinctive facial dysmorphisms [5]. -

Loss of Skin Elasticity Is Associated with Pulmonary Emphysema, Biomarkers of Inflammation, and Matrix Metalloproteinase Activity in Smokers Michael E
O’Brien et al. Respiratory Research (2019) 20:128 https://doi.org/10.1186/s12931-019-1098-7 RESEARCH Open Access Loss of skin elasticity is associated with pulmonary emphysema, biomarkers of inflammation, and matrix metalloproteinase activity in smokers Michael E. O’Brien1, Divay Chandra1, Robert C. Wilson1, Chad M. Karoleski1, Carl R. Fuhrman2, Joseph K. Leader2, Jiantao Pu2, Yingze Zhang1, Alison Morris1,3, Seyed Nouraie1, Jessica Bon1,4, Zsolt Urban5 and Frank C. Sciurba1* Abstract Background: Elastin breakdown and the resultant loss of lung elastic recoil is a hallmark of pulmonary emphysema in susceptible individuals as a consequence of tobacco smoke exposure. Systemic alterations to the synthesis and degradation of elastin may be important to our understanding of disease phenotypes in chronic obstructive pulmonary disease. We investigated the association of skin elasticity with pulmonary emphysema, obstructive lung disease, and blood biomarkers of inflammation and tissue protease activity in tobacco-exposed individuals. Methods: Two hundred and thirty-six Caucasian individuals were recruited into a sub-study of the University of Pittsburgh Specialized Center for Clinically Orientated Research in chronic obstructive pulmonary disease, a prospective cohort study of current and former smokers. The skin viscoelastic modulus (VE), a determinant of skin elasticity, was recorded from the volar forearm and facial wrinkling severity was determined using the Daniell scoring system. Results: In a multiple regression analysis, reduced VE was significantly associated with cross-sectional measurement of airflow obstruction (FEV1/FVC) and emphysema quantified from computed tomography (CT) images, β = 0.26, p = 0.001 and β = 0.24, p = 0.001 respectively. -

Scoliosis in Paraplegia
Paraplegia (1974), II, 290-292 SCOLIOSIS IN PARAPLEGIA By JOHN A. ODOM, JR., M.D. and COURTN EY W. BROWN, M.D. Children's Hospital, Denver, Colorado in conjunction with ROBERT R. JACKSON, M.D., HARRY R. HA HN, M.D. and TERRY V. CARLE, M.D. Craig Rehabilitation Hospital, Englewood, Colorado WITH the increasing instance of excellent medical care, more children with traumatic paraplegia and myelomeningocele with paraplegia live to adulthood. In these two groups of patients there is a high instance of scoliosis, kyphosis and lordosis. Much attention in the past years has been placed on hips and feet but only in the last decade has there been much attention concentrated on the treatment of the spines of these patients. Most of this attention has been toward the patient with a traumatic paraplegia with development of scoliosis. Some attempts have been made at fusing the scoliotic spine of myelomeningo celes by Harrington Instrumentation but with a rather high instance of complica tions and failures. With the event of anterior instrumentation by Dr. Alan Dwyer of Sydney, Australia, the anterior approach to the spine is becoming widely accepted and very successfully used. This is especially valuable in the correction and fusion of the spine with no posterior elements from birth. MATERIAL FOR STUDY Between March of 1971 and June of 1973, there have been 26 paraplegics with scoliosis who have been cared for by the authors. All of these patients have either been myelomeningoceles with paraplegia or spinal cord injuries, all of whom have had scoliosis, kyphosis, or severe lordosis. -

An Unusual Cause of Back Pain in Osteoporosis: Lessons from a Spinal Lesion
Ann Rheum Dis 1999;58:327–331 327 MASTERCLASS Series editor: John Axford Ann Rheum Dis: first published as 10.1136/ard.58.6.327 on 1 June 1999. Downloaded from An unusual cause of back pain in osteoporosis: lessons from a spinal lesion S Venkatachalam, Elaine Dennison, Madeleine Sampson, Peter Hockey, MIDCawley, Cyrus Cooper Case report A 77 year old woman was admitted with a three month history of worsening back pain, malaise, and anorexia. On direct questioning, she reported that she had suVered from back pain for four years. The thoracolumbar radiograph four years earlier showed T6/7 vertebral collapse, mild scoliosis, and degenerative change of the lumbar spine (fig 1); but other investigations at that time including the eryth- rocyte sedimentation rate (ESR) and protein electophoresis were normal. Bone mineral density then was 0.914 g/cm2 (T score = −2.4) at the lumbar spine, 0.776 g/cm2 (T score = −1.8) at the right femoral neck and 0.738 g/cm2 (T score = −1.7) at the left femoral neck. She was given cyclical etidronate after this vertebral collapse as she had suVered a previous fragility fracture of the left wrist. On admission, she was afebrile, but general examination was remarkable for pallor, dental http://ard.bmj.com/ caries, and cellulitis of the left leg. A pansysto- lic murmur was heard at the cardiac apex on auscultation; there were no other signs of bac- terial endocarditis. She had kyphoscoliosis and there was diVuse tenderness of the thoraco- lumbar spine. Her neurological examination was unremarkable. on September 29, 2021 by guest. -
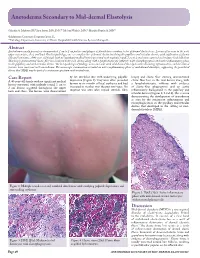
Anetoderma Secondary to Mid-Dermal Elastolysis
Anetoderma Secondary to Mid-dermal Elastolysis Gabriela A. Maloney, BS,* Jane James, MD, PhD,** Michael Welsch, MD,** Marylee Braniecki, MD** *Midwestern University, Downers Grove, IL **Pathology Department, University of Illinois Hospital & Health Sciences System, Chicago, IL Abstract Anetoderma usually presents as circumscribed, 1 cm to 2 cm patches and plaques of flaccid skin secondary to loss of dermal elastic tissue. Lesions often occur in the neck, upper extremities, chest, and back. On histopathology, one sees complete loss of dermal elastin involving the papillary and reticular dermis, with infiltration of plasma cells and histiocytes. A 40-year-old female with no significant medical history presented with multiple round, 1 cm to 2 cm lesions scattered on her upper back and chest. Skin biopsy demonstrated elastic-fiber loss localized to the mid-dermis along with a lymphohistiocytic infiltrate with elastophagocytosis and active inflammatory phase in the papillary and mid-reticular dermis. The histopathological findings were consistent with mid-dermal elastolysis with advancing inflammation, and the clinical features were consistent with anetoderma. The microscopic examination revealed an active inflammatory phase of mid-dermal elastolysis, supporting the postulated theory that MDE may be part of a continuous spectrum with anetoderma. Case Report by lax, wrinkled skin with underlying palpable biopsy and elastic-fiber staining demonstrated A 40-year-old female with no significant medical depression (Figure 1). They were often preceded elastic-fiber loss in the mid-dermis along with history presented with multiple round, 1 cm to by two to six months of local erythema and had a lymphohistiocytic infiltrate with evidence 2 cm lesions scattered throughout the upper increased in number over the past two years. -

Sotos Syndrome
European Journal of Human Genetics (2007) 15, 264–271 & 2007 Nature Publishing Group All rights reserved 1018-4813/07 $30.00 www.nature.com/ejhg PRACTICAL GENETICS In association with Sotos syndrome Sotos syndrome is an autosomal dominant condition characterised by a distinctive facial appearance, learning disability and overgrowth resulting in tall stature and macrocephaly. In 2002, Sotos syndrome was shown to be caused by mutations and deletions of NSD1, which encodes a histone methyltransferase implicated in chromatin regulation. More recently, the NSD1 mutational spectrum has been defined, the phenotype of Sotos syndrome clarified and diagnostic and management guidelines developed. Introduction In brief Sotos syndrome was first described in 1964 by Juan Sotos Sotos syndrome is characterised by a distinctive facial and the major diagnostic criteria of a distinctive facial appearance, learning disability and childhood over- appearance, childhood overgrowth and learning disability growth. were established in 1994 by Cole and Hughes.1,2 In 2002, Sotos syndrome is associated with cardiac anomalies, cloning of the breakpoints of a de novo t(5;8)(q35;q24.1) renal anomalies, seizures and/or scoliosis in B25% of translocation in a child with Sotos syndrome led to the cases and a broad variety of additional features occur discovery that Sotos syndrome is caused by haploinsuffi- less frequently. ciency of the Nuclear receptor Set Domain containing NSD1 abnormalities, such as truncating mutations, protein 1 gene, NSD1.3 Subsequently, extensive analyses of missense mutations in functional domains, partial overgrowth cases have shown that intragenic NSD1 muta- gene deletions and 5q35 microdeletions encompass- tions and 5q35 microdeletions encompassing NSD1 cause ing NSD1, are identifiable in the majority (490%) of 490% of Sotos syndrome cases.4–10 In addition, NSD1 Sotos syndrome cases. -

De Barsy Syndrome
orphananesthesia Anesthesia recommendations for patients suffering from De Barsy syndrome Disease name: De Barsy syndrome ICD 10: Q87.7; OMIM 614438 Synonyms: DBS, De Barsy-Moens-Dierckx syndrome, Progeroid syndrome of De Barsy, Autosomal recessive cutis laxa Type 3 *With 2 gene subdivisions: ARCL3A: caused by a ALDH18A1 mutation ARCL3B: caused by a PYCR1 mutation DeBarsy syndrome is a rare clinical syndrome characterized by cutis laxa, ophthalmic opacification, skeletal malformations, as well as mental and growth retardation. This disease is genetically transmitted in an autosomal recessive fashion. Affected patients often require surgical correction of ophthalmic and orthopaedic abnormalities. This syndrome was first described by A.M. De Barsy in 1967 and less than 100 known cases are documented in the medical literature. Very little has been published on this rare disorder and only a single article has addressed anesthesia case outcomes and management strategies (Aponte, 2010). The diverse collection of clinical manifestations in De Barsy syndrome includes: intra-uterine growth retardation (IUGR), postnatal growth delay, motor delay, cognitive impairment, hypontonia, athetoid movements, malformations, microcephaly, wormian bones, large fontanelles, facial dysmorphism, cataracts, corneal clouding, thin/wrinkled skin, easy bruising, sparse hair, joint laxity, osteopenia, and inguinal hernias. Medicine in progress Perhaps new knowledge Every patient is unique Perhaps the diagnostic is wrong Find more information on the disease, its centres