Cladistic Analysis of the Atypoides Plus Antrodiaetus Lineage of Mygalomorph Spiders (Araneae, Antrodiaetidae )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogeography of the Caribbean Cyrtognatha Spiders Klemen Čandek1,6,7, Ingi Agnarsson2,4, Greta J
www.nature.com/scientificreports OPEN Biogeography of the Caribbean Cyrtognatha spiders Klemen Čandek1,6,7, Ingi Agnarsson2,4, Greta J. Binford3 & Matjaž Kuntner 1,4,5,6 Island systems provide excellent arenas to test evolutionary hypotheses pertaining to gene fow and Received: 23 July 2018 diversifcation of dispersal-limited organisms. Here we focus on an orbweaver spider genus Cyrtognatha Accepted: 1 November 2018 (Tetragnathidae) from the Caribbean, with the aims to reconstruct its evolutionary history, examine Published: xx xx xxxx its biogeographic history in the archipelago, and to estimate the timing and route of Caribbean colonization. Specifcally, we test if Cyrtognatha biogeographic history is consistent with an ancient vicariant scenario (the GAARlandia landbridge hypothesis) or overwater dispersal. We reconstructed a species level phylogeny based on one mitochondrial (COI) and one nuclear (28S) marker. We then used this topology to constrain a time-calibrated mtDNA phylogeny, for subsequent biogeographical analyses in BioGeoBEARS of over 100 originally sampled Cyrtognatha individuals, using models with and without a founder event parameter. Our results suggest a radiation of Caribbean Cyrtognatha, containing 11 to 14 species that are exclusively single island endemics. Although biogeographic reconstructions cannot refute a vicariant origin of the Caribbean clade, possibly an artifact of sparse outgroup availability, they indicate timing of colonization that is much too recent for GAARlandia to have played a role. Instead, an overwater colonization to the Caribbean in mid-Miocene better explains the data. From Hispaniola, Cyrtognatha subsequently dispersed to, and diversifed on, the other islands of the Greater, and Lesser Antilles. Within the constraints of our island system and data, a model that omits the founder event parameter from biogeographic analysis is less suitable than the equivalent model with a founder event. -

Cryptic Species Delimitation in the Southern Appalachian Antrodiaetus
Cryptic species delimitation in the southern Appalachian Antrodiaetus unicolor (Araneae: Antrodiaetidae) species complex using a 3RAD approach Lacie Newton1, James Starrett1, Brent Hendrixson2, Shahan Derkarabetian3, and Jason Bond4 1University of California Davis 2Millsaps College 3Harvard University 4UC Davis May 5, 2020 Abstract Although species delimitation can be highly contentious, the development of reliable methods to accurately ascertain species boundaries is an imperative step in cataloguing and describing Earth's quickly disappearing biodiversity. Spider species delimi- tation remains largely based on morphological characters; however, many mygalomorph spider populations are morphologically indistinguishable from each other yet have considerable molecular divergence. The focus of our study, Antrodiaetus unicolor species complex which contains two sympatric species, exhibits this pattern of relative morphological stasis with considerable genetic divergence across its distribution. A past study using two molecular markers, COI and 28S, revealed that A. unicolor is paraphyletic with respect to A. microunicolor. To better investigate species boundaries in the complex, we implement the cohesion species concept and employ multiple lines of evidence for testing genetic exchangeability and ecological interchange- ability. Our integrative approach includes extensively sampling homologous loci across the genome using a RADseq approach (3RAD), assessing population structure across their geographic range using multiple genetic clustering analyses that include STRUCTURE, PCA, and a recently developed unsupervised machine learning approach (Variational Autoencoder). We eval- uate ecological similarity by using large-scale ecological data for niche-based distribution modeling. Based on our analyses, we conclude that this complex has at least one additional species as well as confirm species delimitations based on previous less comprehensive approaches. -

Final Project Completion Report
CEPF SMALL GRANT FINAL PROJECT COMPLETION REPORT Organization Legal Name: - Tarantula (Araneae: Theraphosidae) spider diversity, distribution and habitat-use: A study on Protected Area adequacy and Project Title: conservation planning at a landscape level in the Western Ghats of Uttara Kannada district, Karnataka Date of Report: 18 August 2011 Dr. Manju Siliwal Wildlife Information Liaison Development Society Report Author and Contact 9-A, Lal Bahadur Colony, Near Bharathi Colony Information Peelamedu Coimbatore 641004 Tamil Nadu, India CEPF Region: The Western Ghats Region (Sahyadri-Konkan and Malnad-Kodugu Corridors). 2. Strategic Direction: To improve the conservation of globally threatened species of the Western Ghats through systematic conservation planning and action. The present project aimed to improve the conservation status of two globally threatened (Molur et al. 2008b, Siliwal et al., 2008b) ground dwelling theraphosid species, Thrigmopoeus insignis and T. truculentus endemic to the Western Ghats through systematic conservation planning and action. Investment Priority 2.1 Monitor and assess the conservation status of globally threatened species with an emphasis on lesser-known organisms such as reptiles and fish. The present project was focused on an ignored or lesser-known group of spiders called Tarantulas/ Theraphosid spiders and provided valuable information on population status and potential conservation sites in Uttara Kannada district, which will help in future monitoring and assessment of conservation status of the two globally threatened theraphosid species T. insignis and Near Threatened T. truculentus. Investment Priority 2.3. Evaluate the existing protected area network for adequate globally threatened species representation and assess effectiveness of protected area types in biodiversity conservation. -

Tarantulas in the Pacific Northwest1
WSU Puyallup REC PLS-108 Updated July 2003 Tarantulas in the Pacific Northwest1 Tarantulas (Fig. 1) in the Pacific Northwest? Well, maybe not like the hairy monsters of the tropics, but some very interesting "atypical" species do occur here. Our species belong to the family Antrodiaetidae. One of our most common spiders is the folding-door spider, Antrodiaetus pacificus (Simon). It is a fairly large species, females ranging from 11 to 13 millimeters in length, males slightly smaller. They are generally dark brown to almost black in color with the abdomen purplish brown. Males are characterized by their long legs, slim bodies, and three tergites (hardened plates) on the abdomen. Females (Fig. 2) are more robust with only one tergite. These spiders excavate burrows in the soil or in damp, rotten wood, digging with a row of spines on each chelicer, known as a ratellum. The six to ten inch deep vertical shafts are lined with silk. The webbing extends beyond ground level as a short collar of camouflaged silk. The turret’s two sides may be drawn in by the occupant, forming two "doors" which meet in the middle. At night, Antrodiaetus assumes a foraging posture with its pedipalps and first pair of legs just touching the rim of silk at the mouth of the tube. In this position, the folding door spider can readily detect an insect moving above ground. The spider will leap out of its burrow with lightning speed, seize its victim, and drop back down, like a terrorizing Jack-in-the-box. When finished with its meal, it will add the insect's dry, dismembered body to a silk-covered trash pile at the bottom of its burrow. -
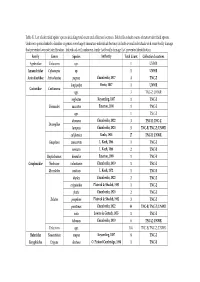
Table S1. List of Identified Spider Species Including Total Count and Collection Locations. Bold Cells Include Counts of Mature Identified Species
Table S1. List of identified spider species including total count and collection locations. Bold cells include counts of mature identified species. Unknown species linked to families or genera were largely immature individuals but may include several individuals with some bodily damage that prevented accurate identification. Individuals with unknown family had bodily damage that prevented identification. Family Genus Species Authority Total Count Collection Locations Agelenidae Unknown spp. 1 UNWR Amaurobiidae Cybaeopsis sp. 1 UNWR Antrodiaetidae Antrodiaetus pugnax Chamberlin, 1917 4 TNC-Z longipalpa Hentz, 1847 1 UNWR Corinnidae Castianeira spp. 3 TNC-Z; UNWR neglectus Keyserling, 1887 1 TNC-Z Drassodes saccatus Emerton, 1890 1 TNC-Z spp. 1 TNC-Z dromeus Chamberlin, 1922 3 TNC-B; TNC-Z Drassyllus lamprus Chamberlin, 1920 3 TNC-B; TNC-Z; UNWR californica Banks, 1904 17 TNC-B; UNWR Gnaphosa muscorum L. Koch, 1866 1 TNC-Z sericata L. Koch, 1866 2 TNC-B Haplodrassus hiemalis Emerton, 1909 1 TNC-B Gnaphosidae Nodocion voluntaries Chamberlin, 1919 1 TNC-Z Urozelotes rusticus L. Koch, 1872 1 TNC-B duplex Chamberlin, 1922 2 TNC-Z exiguioides Platnick & Shadab, 1983 1 TNC-Z fratis Chamberlin, 1920 2 TNC-Z Zelotes josephine Platnick & Shadab, 1983 3 TNC-Z puritanus Chamberlin, 1922 44 TNC-B; TNC-Z; UNWR sula Lowrie & Gertsch, 1955 1 TNC-Z tubuous Chamberlin, 1919 6 TNC-Z; UNWR Unknown spp. 184 TNC-B; TNC-Z; UNWR Hahniidae Neoantistea magna Keyserling, 1887 8 TNC-Z Linyphiidae Erigone dentosa O. Pickard-Cambridge, 1894 1 TNC-B spp. 1 TNC-Z Unknown spp. 13 TNC-Z; UNWR mccooki Montgomery, 1904 95 TNC-B; TNC-Z; UNWR Schizocosa minnesotensis Gertsch, 1934 25 TNC-B Lycosidae spp. -

The Effects of Native and Non-Native Grasses on Spiders, Their Prey, and Their Interactions
Spiders in California’s grassland mosaic: The effects of native and non-native grasses on spiders, their prey, and their interactions by Kirsten Elise Hill A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Environmental Science, Policy, and Management in the GRADUATE DIVISION of the University of California, Berkeley Committee in charge: Professor Joe R. McBride, Chair Professor Rosemary G. Gillespie Professor Mary E. Power Spring 2014 © 2014 Abstract Spiders in California’s grassland mosaic: The effects of native and non-native grasses on spiders, their prey, and their interactions by Kirsten Elise Hill Doctor of Philosophy in Environmental Science and Policy Management University of California, Berkeley Professor Joe R. McBride, Chair Found in nearly all terrestrial ecosystems, small in size and able to occupy a variety of hunting niches, spiders’ consumptive effects on other arthropods can have important impacts for ecosystems. This dissertation describes research into spider populations and their interactions with potential arthropod prey in California’s native and non-native grasslands. In meadows found in northern California, native and non-native grassland patches support different functional groups of arthropod predators, sap-feeders, pollinators, and scavengers and arthropod diversity is linked to native plant diversity. Wandering spiders’ ability to forage within the meadow’s interior is linked to the distance from the shaded woodland boundary. Native grasses offer a cooler conduit into the meadow interior than non-native annual grasses during midsummer heat. Juvenile spiders in particular, are more abundant in the more structurally complex native dominated areas of the grassland. -

Phase I Environmental Site Assessment Report Record No
Phase I Environmental Site Assessment Report Record No. 101650317 17771-17789 Panama City Beach Parkway; 17690 Front Beach Road Panama City Beach, FL 32413 Prepared For: Federal Deposit Insurance Corporation (FDIC) as Receiver for Peoples First Community Bank,Bank No. 10165, c/o CBRE 2001 Ross Avenue, 33rd Floor Dallas, TX 75201 Prepared By: Tetra Tech, Inc. 17885 Von Karman Avenue Irvine, CA 92614 TETRA TECH PROJECT T24023.003 2010-08-25 17885 Von Karman Avenue, Suite 500 Irvine, CA 92614 Office: (949) 809-5000 Fax: (949) 809-5010 August 25, 2010 Mr. Jon Walker (CB Richard Ellis, Inc. [CBRE]) Federal Deposit Insurance Corporation (FDIC) as Receiver for Peoples First Community Bank, Bank No. 10165 c/o CBRE 2001 Ross Avenue, 33rd Floor Dallas, TX 75201 RE: Phase I Environmental Site Assessment Record No. 101650317 17771-17789 Panama City Parkway and 17690 Front Beach Road Panama City Beach, Florida 32413 Project No. T24023.003 Dear Mr. Walker: Tetra Tech, Inc. (Tetra Tech) is pleased to submit this Phase I Environmental Site Assessment (ESA) report to Federal Deposit Insurance Corporation (FDIC), as Receiver for Peoples First Community Bank, Bank No. 10165, c/o CBRE, for the above-referenced property (the Site). Tetra Tech found one recognized environmental conditions (RECs), no historical RECs (HRECs), no potential environmental concerns (PECs), and three business environmental risks (BERs) in connection with the Site. It is Tetra Tech’s understanding that this ESA is being requested in conjunction with due diligence activities for the Site by the FDIC, as Receiver for Peoples First Community Bank, Bank No. -

Species Are Hypotheses: Avoid Connectivity Assessments Based on Pillars of Sand Eric Pante, Nicolas Puillandre, Amélia Viricel, Sophie Arnaud-Haond, D
Species are hypotheses: avoid connectivity assessments based on pillars of sand Eric Pante, Nicolas Puillandre, Amélia Viricel, Sophie Arnaud-Haond, D. Aurelle, Magalie Castelin, Anne Chenuil, Christophe Destombe, Didier Forcioli, Myriam Valero, et al. To cite this version: Eric Pante, Nicolas Puillandre, Amélia Viricel, Sophie Arnaud-Haond, D. Aurelle, et al.. Species are hypotheses: avoid connectivity assessments based on pillars of sand. Molecular Ecology, Wiley, 2015, 24 (3), pp.525-544. hal-02002440 HAL Id: hal-02002440 https://hal.archives-ouvertes.fr/hal-02002440 Submitted on 31 Jan 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Molecular Ecology Species are hypotheses : avoid basing connectivity assessments on pillars of sand. Journal:For Molecular Review Ecology Only Manuscript ID: Draft Manuscript Type: Invited Reviews and Syntheses Date Submitted by the Author: n/a Complete List of Authors: Pante, Eric; UMR 7266 CNRS - Université de La Rochelle, Puillandre, Nicolas; MNHN, Systematique & Evolution Viricel, Amélia; UMR 7266 CNRS - -

Phylogenomic Analysis and Revised Classification of Atypoid Mygalomorph Spiders (Araneae, Mygalomorphae), with Notes on Arachnid Ultraconserved Element Loci
Phylogenomic analysis and revised classification of atypoid mygalomorph spiders (Araneae, Mygalomorphae), with notes on arachnid ultraconserved element loci Marshal Hedin1, Shahan Derkarabetian1,2,3, Adan Alfaro1, Martín J. Ramírez4 and Jason E. Bond5 1 Department of Biology, San Diego State University, San Diego, CA, United States of America 2 Department of Biology, University of California, Riverside, Riverside, CA, United States of America 3 Department of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, Cambridge, MA, United States of America 4 Division of Arachnology, Museo Argentino de Ciencias Naturales ``Bernardino Rivadavia'', Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina 5 Department of Entomology and Nematology, University of California, Davis, CA, United States of America ABSTRACT The atypoid mygalomorphs include spiders from three described families that build a diverse array of entrance web constructs, including funnel-and-sheet webs, purse webs, trapdoors, turrets and silken collars. Molecular phylogenetic analyses have generally supported the monophyly of Atypoidea, but prior studies have not sampled all relevant taxa. Here we generated a dataset of ultraconserved element loci for all described atypoid genera, including taxa (Mecicobothrium and Hexurella) key to understanding familial monophyly, divergence times, and patterns of entrance web evolution. We show that the conserved regions of the arachnid UCE probe set target exons, such that it should be possible to combine UCE and transcriptome datasets in arachnids. We also show that different UCE probes sometimes target the same protein, and under the matching parameters used here show that UCE alignments sometimes include non- Submitted 1 February 2019 orthologs. Using multiple curated phylogenomic matrices we recover a monophyletic Accepted 28 March 2019 Published 3 May 2019 Atypoidea, and reveal that the family Mecicobothriidae comprises four separate and divergent lineages. -

Folding Door Spider
Folding Door Spider Order: Arachnida Family: Antrodiaetidae Genus/species: Antrodiaetus species Description: The folding door spider is tan to chestnut brown about ¼” – 1 ½ “long. They have rakelike rows of spines on each jaw, or chelicera, which they use for digging. They have 3 claws on each foot. Folding door spiders have 1-4 small crescent shaped sclerites (hardened plates) on the top of the abdomen. They have 6 eyes in two groups of three. Habitat: Folding door spiders excavate burrows in the ground that they line with silk. At the entrance they construct a collapsible “trap door”. When active they open their trapdoor at night and wait just inside the entrance to strike at passing prey. The spider detects the prey by sensing vibrations. When mature, males abandon their burrows during rainy periods and wander in search of females. Life Cycle: Most spiders live 1 to 2 years. The adult male begins courtship after his palp is filled with sperm and he has found a female. Soon after mating the male dies. After a week or more the mated female deposits her eggs in a silken sac. Weeks later, or sometimes not until the following spring, the young spiderlings emerge. Economic Importance & Management: All spiders are voracious predators. Folding Door spiders feed on insects, and are considered highly beneficial because they keep the burgeoning insect populations in check. References: The Audubon Society Field Guide to NA Lorus & Marjery Milne Spiders! Usually helpful, sometimes a nuisance, Jean Natter Spiders of NA-identification manual, D. Ubick, P. Paquin P.E .Cushing and V. -

The Mygalomorph Spider Genus Atypoides
THE MYGALOMORPH SPIDER GENUS A TYPOIDES (ARANEAE ANTRODIAETIDAE)* BY FREDERICK _,it. COYLE Biological Laboratories, Harvard University INTRODUCTION The genus ztt:vpoides belongs to the mygalomorph spider amily Antrodiaetidae, the remainder of which I am presently revising, and is closely related to the much larger genus A ntrodiaetus. Zltypoides was established by O. P.-Cambridge (I883) with his description of A typoides riversi. Since then two additional species, have been discovered. The genus is solely Nearctic and has a markedly dis- junct continental distribution, with two. species in northern Cali- fornia and southern Oregon and one in southern Illinois and Missouri. Like the other species of antrodiaetids, those o.f A typoides live in tubular silk-lined burrows in the ground and nocturnally capture prey which come within reach of the burrow entrance. In the present revision A typoides riversi is redescribed, the two new species are described, and the geographic variation of each species is analyzed. Information on the ecology, life history, and behavior of these species will be included in a future paper treating the entire family. Acknowledgements. I am indebted to Dr. H. W. Levi of the Museum of Comparative Zoology for encouragement and advice and to Dr. W. J. Gertsch of the American Museum of Natural History for his suggestion that I study this genus and for the large loan of A.M.N.H. material, mu,ch of which he himself has collected. Mr. J. A. Beatty, Mr. Patrick Craig, the California Academy of Sciences, and the University of Kansas have each loaned specimens. I am grateful to Prof. -

Multilocus Species Delimitation in a Complex of Morphologically Conserved Trapdoor Spiders (Mygalomorphae, Antrodiaetidae, Aliatypus)
Syst. Biol. 62(6):805–823, 2013 © The Author(s) 2013. Published by Oxford University Press, on behalf of the Society of Systematic Biologists. All rights reserved. For Permissions, please email: [email protected] DOI:10.1093/sysbio/syt041 Advance Access publication June 13, 2013 Multilocus Species Delimitation in a Complex of Morphologically Conserved Trapdoor Spiders (Mygalomorphae, Antrodiaetidae, Aliatypus) ,∗ JORDAN D. SATLER1 ,BRYAN C. CARSTENS1, AND MARSHAL HEDIN2 1Department of Evolution, Ecology and Organismal Biology, The Ohio University, Columbus, OH 43210, USA and 2Department of Biology, San Diego University, San Diego, CA 92182, USA ∗ Correspondence to be sent to: Department of Evolution, Ecology and Organismal Biology, 318 West 12th Avenue, The Ohio State University, Columbus, OH 43210, USA; E-mail: [email protected] Received 17 December 2012; reviews returned 4 March 2013; accepted 7 June 2013 Associate Editor: Robb Brumfield Abstract.—Species are a fundamental unit for biological studies, yet no uniform guidelines exist for determining species limits in an objective manner. Given the large number of species concepts available, defining species can be both highly Downloaded from subjective and biased. Although morphology has been commonly used to determine species boundaries, the availability and prevalence of genetic data has allowed researchers to use such data to make inferences regarding species limits. Genetic data also have been used in the detection of cryptic species, where other lines of evidence (morphology in particular) may underestimate species diversity. In this study, we investigate species limits in a complex of morphologically conserved trapdoor spiders (Mygalomorphae, Antrodiaetidae, Aliatypus) from California. Multiple approaches were used to determine species boundaries in this highly genetically fragmented group, including both multilocus discovery and validation http://sysbio.oxfordjournals.org/ approaches (plus a chimeric approach).