Gifc-2018 Book of Abstracts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flexible Fluidic Actuators for Soft Robotic Applications
University of Wollongong Research Online University of Wollongong Thesis Collection 2017+ University of Wollongong Thesis Collections 2019 Flexible Fluidic Actuators for Soft Robotic Applications Weiping Hu Follow this and additional works at: https://ro.uow.edu.au/theses1 University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Research Online is the open access institutional repository for the University of -

Environmentally Controlled Curvature of Single Collagen Proteins
Environmentally controlled curvature of single collagen proteins Naghmeh Rezaei, Aaron Lyons and Nancy R. Forde* Department of Physics Simon Fraser University 8888 University Drive Burnaby, BC V5A 1S6 CANADA *Corresponding author. 778-782-3161. [email protected]. ORCID: 0000-0002-5479-7073 - 1 - ABSTRACT The predominant structural protein in vertebrates is collagen, which plays a key role in extracellular matrix and connective tissue mechanics. Despite its prevalence and physical importance in biology, the mechanical properties of molecular collagen are far from established. The flexibility of its triple helix is unresolved, with descriptions from different experimental techniques ranging from flexible to semirigid. Furthermore, it is unknown how collagen type (homo- vs. heterotrimeric) and source (tissue-derived vs. recombinant) influence flexibility. Using SmarTrace, a chain tracing algorithm we devised, we performed statistical analysis of collagen conformations collected with atomic force microscopy (AFM) to Our results show that types I, II and III collagens the key fibrillar varieties exhibit molecular flexibilities that are very similar. However, collagen conformations are strongly modulated by salt, transitioning from compact to extended as KCl concentration increases, in both neutral and acidic pH. While analysis with a standard worm-like chain model suggests that the persistence length of collagen can attain almost any value within the literature range, closer inspection reveals that this modulation to changes in flexibility, but rather arises from the induction of curvature (either intrinsic or induced by interactions with the mica surface). By modifying standard polymer theory to include innate curvature, we show that collagen behaves as an equilibrated curved worm-like chain (cWLC) in two dimensions. -

Peptoid Residues Make Diverse, Hyperstable Collagen Triple Helices
Peptoid Residues Make Diverse, Hyperstable Collagen Triple Helices Julian L. Kessler1, Grace Kang1, Zhao Qin2, Helen Kang1, Frank G. Whitby3, Thomas E. Cheatham III4, Christopher P. Hill3, Yang Li1,*, and S. Michael Yu1,5 1Department of Biomedical Engineering, University of Utah, Salt Lake City, Utah 84112, USA 2Department of Civil & Environmental Engineering, Collagen of Engineering & Computer Science, Syracuse University, Syracuse, New York 13244, USA 3Department of Biochemistry, University of Utah School of Medicine, Salt Lake City, UT 84112, USA 4Department of Medicinal Chemistry, College of Pharmacy, L. S. Skaggs Pharmacy Research Institute, University of Utah, Salt Lake City, Utah 84112, USA 5Department of Pharmaceutics and Pharmaceutical Chemistry, University of Utah, Salt Lake City, Utah 84112, USA *Corresponding Author: Yang Li ([email protected]) Abstract The triple-helical structure of collagen, responsible for collagen’s remarkable biological and mechanical properties, has inspired both basic and applied research in synthetic peptide mimetics for decades. Since non-proline amino acids weaken the triple helix, the cyclic structure of proline has been considered necessary, and functional collagen mimetic peptides (CMPs) with diverse sidechains have been difficult to produce. Here we show that N-substituted glycines (N-glys), also known as peptoid residues, exhibit a general triple-helical propensity similar to or greater than proline, allowing synthesis of thermally stable triple-helical CMPs with unprecedented sidechain diversity. We found that the N-glys stabilize the triple helix by sterically promoting the preorganization of individual CMP chains into the polyproline-II helix conformation. Our findings were supported by the crystal structures of two atomic-resolution N-gly-containing CMPs, as well as experimental and computational studies spanning more than 30 N-gly-containing peptides. -

How Influenza Virus Uses Host Cell Pathways During Uncoating
cells Review How Influenza Virus Uses Host Cell Pathways during Uncoating Etori Aguiar Moreira 1 , Yohei Yamauchi 2 and Patrick Matthias 1,3,* 1 Friedrich Miescher Institute for Biomedical Research, 4058 Basel, Switzerland; [email protected] 2 Faculty of Life Sciences, School of Cellular and Molecular Medicine, University of Bristol, Bristol BS8 1TD, UK; [email protected] 3 Faculty of Sciences, University of Basel, 4031 Basel, Switzerland * Correspondence: [email protected] Abstract: Influenza is a zoonotic respiratory disease of major public health interest due to its pan- demic potential, and a threat to animals and the human population. The influenza A virus genome consists of eight single-stranded RNA segments sequestered within a protein capsid and a lipid bilayer envelope. During host cell entry, cellular cues contribute to viral conformational changes that promote critical events such as fusion with late endosomes, capsid uncoating and viral genome release into the cytosol. In this focused review, we concisely describe the virus infection cycle and highlight the recent findings of host cell pathways and cytosolic proteins that assist influenza uncoating during host cell entry. Keywords: influenza; capsid uncoating; HDAC6; ubiquitin; EPS8; TNPO1; pandemic; M1; virus– host interaction Citation: Moreira, E.A.; Yamauchi, Y.; Matthias, P. How Influenza Virus Uses Host Cell Pathways during 1. Introduction Uncoating. Cells 2021, 10, 1722. Viruses are microscopic parasites that, unable to self-replicate, subvert a host cell https://doi.org/10.3390/ for their replication and propagation. Despite their apparent simplicity, they can cause cells10071722 severe diseases and even pose pandemic threats [1–3]. -

Ebolaviruses: New Roles for Old Proteins
REVIEW Ebolaviruses: New roles for old proteins Diego Cantoni, Jeremy S. Rossman* School of Biosciences, University of Kent, Canterbury, United Kingdom * [email protected] Abstract In 2014, the world witnessed the largest Ebolavirus outbreak in recorded history. The subse- quent humanitarian effort spurred extensive research, significantly enhancing our under- standing of ebolavirus replication and pathogenicity. The main functions of each ebolavirus protein have been studied extensively since the discovery of the virus in 1976; however, the recent expansion of ebolavirus research has led to the discovery of new protein functions. a1111111111 These newly discovered roles are revealing new mechanisms of virus replication and patho- a1111111111 genicity, whilst enhancing our understanding of the broad functions of each ebolavirus viral a1111111111 a1111111111 protein (VP). Many of these new functions appear to be unrelated to the protein's primary a1111111111 function during virus replication. Such new functions range from bystander T-lymphocyte death caused by VP40-secreted exosomes to new roles for VP24 in viral particle formation. This review highlights the newly discovered roles of ebolavirus proteins in order to provide a more encompassing view of ebolavirus replication and pathogenicity. OPEN ACCESS Citation: Cantoni D, Rossman JS (2018) Ebolaviruses: New roles for old proteins. PLoS Negl Trop Dis 12(5): e0006349. https://doi.org/ Author summary 10.1371/journal.pntd.0006349 Between 2014 and 2016, West Africa experienced the largest Ebolavirus outbreak in Editor: Patricia V. Aguilar, University of Texas recorded history. The international containment effort spurred extensive research that is Medical Branch, UNITED STATES enhancing our understanding of ebolavirus replication and pathogenicity. -
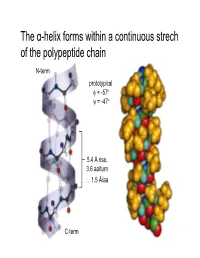
The Α-Helix Forms Within a Continuous Strech of the Polypeptide Chain
The α-helix forms within a continuous strech of the polypeptide chain N-term prototypical φ = -57 ° ψ = -47 ° 5.4 Å rise, 3.6 aa/turn ∴ 1.5 Å/aa C-term α-Helices have a dipole moment, due to unbonded and aligned N-H and C=O groups β-Sheets contain extended (β-strand) segments from separate regions of a protein prototypical φ = -139 °, ψ = +135 ° prototypical φ = -119 °, ψ = +113 ° (6.5Å repeat length in parallel sheet) Antiparallel β-sheets may be formed by closer regions of sequence than parallel Beta turn Figure 6-13 The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation • Interactions between adjacent R-groups – Ionic attraction or repulsion – Steric hindrance of adjacent bulky groups Helix wheel The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation • Interactions between adjacent R-groups – Ionic attraction or repulsion – Steric hindrance of adjacent bulky groups • Occurrence of proline and glycine • Interactions between ends of helix and aa R-groups His Glu N-term C-term -

Eesha Acharya Project #1
Eesha Acharya Project #1 Completed Project, Science, Health and Medical Measuring Vitamin C Levels in Cooked Foods Most people know that raw foods contain the most nutrients. However, many people prefer eating cooked foods. the problem is vitamin C is a water-soluble vitamin, so when foods are cooked they lose a lot of this essential nutrient. The purpose of this research is to determine which cooking method best retains the most vitamin C in vegetables. The raw vegetable vitamin C information will be compared to that of other cooking methods (grilling, boiling, and steaming) of that same vegetable. tomatoes, brussel sprouts, kale, bell peppers, broccoli, peas, and a tincture of iodine solution, 2-7% elemental iodine, will be used to test the vitamin C content. The food will be tested by mixing 10g of food to a starch-water mixture and straining the water. Drops of iodine will be added to the strained water until the solution turns black. The more iodine added, means the more vitamin C is in the food. Then the number of drops will be divided by 10g of food. This gives the drops per gram of food. This number will be multiplied by the factor. The drops per gram multiplied by the factor equals mg of vitamin C per gram of food This study is designed to help people consume more vitamin C. Many people in the United States have a vitamin C deficiency. Vitamin C is a key that prevents immune system deficiency and cardiovascular disease. So if a proper cooking method can be found, then people can consume more Vitamin C. -

Y. Ma, X. Feng, J.A. Rogers, Y. Huang and Yihui
Lab on a Chip View Article Online CRITICAL REVIEW View Journal | View Issue Design and application of ‘J-shaped’ stress–strain Cite this: Lab Chip,2017,17,1689 behavior in stretchable electronics: a review Yinji Ma,ab Xue Feng,a John A. Rogers,c Yonggang Huangb and Yihui Zhang *a A variety of natural biological tissues (e.g., skin, ligaments, spider silk, blood vessel) exhibit ‘J-shaped’ stress–strain behavior, thereby combining soft, compliant mechanics and large levels of stretchability, with anatural‘strain-limiting’ mechanism to prevent damage from excessive strain. Synthetic materials with similar stress–strain behaviors have potential utility in many promising applications, such as tissue engineer- ing (to reproduce the nonlinear mechanical properties of real biological tissues) and biomedical devices (to enable natural, comfortable integration of stretchable electronics with biological tissues/organs). Recent advances in this field encompass developments of novel material/structure concepts, fabrication ap- proaches, and unique device applications. This review highlights five representative strategies, including de- signs that involve open network, wavy and wrinkled morphologies, helical layouts, kirigami and origami constructs, and textile formats. Discussions focus on the underlying ideas, the fabrication/assembly routes, Received 18th March 2017, and the microstructure–property relationships that are essential for optimization of the desired ‘J-shaped’ Accepted 21st April 2017 stress–strain responses. Demonstration applications provide examples of the use of these designs in de- formable electronics and biomedical devices that offer soft, compliant mechanics but with inherent robust- DOI: 10.1039/c7lc00289k ness against damage from excessive deformation. We conclude with some perspectives on challenges and rsc.li/loc opportunities for future research. -

Downloads/ Hsp90interactors.Pdf), and Tend to Be Metastable, Being Rapidly Degraded Upon Hsp90 Inhibition
viruses Review Chaperoning the Mononegavirales: Current Knowledge and Future Directions Victor Latorre †, Florian Mattenberger † and Ron Geller * Institute for Integrative Systems Biology (I2SysBio), Universitat de Valencia-CSIC, 46980 Valencia, Spain; [email protected] (V.L.); [email protected] (F.M.) * Correspondence: [email protected]; Tel.: +34-963-543-187 † These authors contributed equally to this work. Received: 16 November 2018; Accepted: 5 December 2018; Published: 8 December 2018 Abstract: The order Mononegavirales harbors numerous viruses of significant relevance to human health, including both established and emerging infections. Currently, vaccines are only available for a small subset of these viruses, and antiviral therapies remain limited. Being obligate cellular parasites, viruses must utilize the cellular machinery for their replication and spread. Therefore, targeting cellular pathways used by viruses can provide novel therapeutic approaches. One of the key challenges confronted by both hosts and viruses alike is the successful folding and maturation of proteins. In cells, this task is faced by cellular molecular chaperones, a group of conserved and abundant proteins that oversee protein folding and help maintain protein homeostasis. In this review, we summarize the current knowledge of how the Mononegavirales interact with cellular chaperones, highlight key gaps in our knowledge, and discuss the potential of chaperone inhibitors as antivirals. Keywords: Mononegavirales; chaperones; antivirals; Hsp70; -

Structure of the Cleavage-Activated Prefusion Form of the Parainfluenza Virus 5 Fusion Protein
Structure of the cleavage-activated prefusion form of the parainfluenza virus 5 fusion protein Brett D. Welcha,b,1, Yuanyuan Liua,b,1, Christopher A. Korsa,b, George P. Lesera,b, Theodore S. Jardetzkyc,2, and Robert A. Lamba,b,2 aHoward Hughes Medical Institute and bDepartment of Molecular Biosciences, Northwestern University, Evanston, IL 60208; and cDepartment of Structural Biology, Stanford University School of Medicine, Stanford, CA 94305 Contributed by Robert A. Lamb, August 9, 2012 (sent for review June 28, 2012) The paramyxovirus parainfluenza virus 5 (PIV5) enters cells by refolding, resulting in formation of a trimeric coiled coil com- fusion of the viral envelope with the plasma membrane through posed of a heptad repeat A region that extends away from the the concerted action of the fusion (F) protein and the receptor viral membrane (18–20). binding protein hemagglutinin-neuraminidase. The F protein folds Peptide inhibitor studies and available atomic structures in- initially to form a trimeric metastable prefusion form that is trig- dicate that many of the key elements of this entry mechanism are gered to undergo large-scale irreversible conformational changes common to other class I viral fusion proteins, such as the hem- to form the trimeric postfusion conformation. It is thought that agglutinin (HA) of influenza virus, gp120/41 of HIV, S protein of F refolding couples the energy released with membrane fusion. severe acute respiratory syndrome coronavirus, and glycoprotein The F protein is synthesized as a precursor (F0) that must be (GP) of Ebola virus (reviewed in ref. 4). Although X-ray struc- cleaved by a host protease to form a biologically active molecule, tures of the six-helix bundle of many type I fusion proteins have F1,F2. -

Flexible Fluidic Actuators for Soft Robotic Applications
University of Wollongong Research Online University of Wollongong Thesis Collection 2017+ University of Wollongong Thesis Collections 2019 Flexible Fluidic Actuators for Soft Robotic Applications Weiping Hu University of Wollongong Follow this and additional works at: https://ro.uow.edu.au/theses1 University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Hu, Weiping, Flexible Fluidic Actuators for Soft Robotic Applications, Doctor of Philosophy thesis, School of Mechanical, Materials, Mechatronic and Biomedical Engineering, University of Wollongong, 2019. https://ro.uow.edu.au/theses1/717 Research Online is the open access institutional repository for the University of Wollongong. -

Daftar Peneliti Yang Belum Mengunggah Laporan Anggaran Dan Laporan Akhir Tahun 2014
LAMPIRAN SURAT NO : 4033/E5.1/PE/2014 DAFTAR PENELITI YANG BELUM MENGUNGGAH LAPORAN ANGGARAN DAN LAPORAN AKHIR TAHUN 2014 NO NAMA NIDN PERGURUAN TINGGI JUDUL SKIM LAPORAN ANGGARAN LAPORAN AKHIR Akademi Analis Kesehatan ANALISIS KUALITAS DAN TINGKAT OKSIDASI MINYAK 1 LISA ANDINA 1123038303 Dosen Pemula belum diunggah belum diunggah Borneo Lestari Banjarbaru GORENG CURAH YANG DIGUNAKAN PADA WARUNG Akademi Analis Kesehatan PengembanganMAKAN SEAFOOD Produk KAKI LIMAHerbal MENGGUNAKAN Terstandar Sebagai 2 FAISAL 0710117901 Dosen Pemula belum diunggah belum diunggah Malang Obat Antimalaria dari Ekstrak daun Tanaman Bunga Akademi Analis Kesehatan PENGARUHmatahari (Helianthus AGONIS PEROXISOME annus) PROLIFERATOR- 3 NUGROHO TRISTYANTO 0713068101 Dosen Pemula belum diunggah sudah diunggah Malang ACTIVATED RECEPTOR γ (PPARγ) TERHADAP PRODUKSI Akademi Analis Kesehatan ISOLASIVASCULAR DAN ENDOTHELIAL ELUSIDASI STRUKTUR GROWTH SENYAWAFACTOR (VEGF) 4 ROIHATUL MUTIAH 0703028003 Pekerti belum diunggah sudah diunggah Malang ANTIKANKER DARI DAUN DAN BUNGA Calotrophis Akademi Analis Kesehatan Pengaruhgigantea SERTAKualitas PENENTUAN Tidur Dengan MEKANISME Jumlah Sel Darah 5 CISILLIA ADHIYANI 0623038004 Dosen Pemula belum diunggah belum diunggah Nasional Surakarta Pekerja Dengan Sistem Shift Akademi Bahasa Asing RA PENGEMBANGAN MODEL RELOKASI 6 AGUS DWIDOSO WARSONO 0626086502 Disertasi Doktor belum diunggah belum diunggah Kartini Surakarta PEDAGANG KAKI LIMA DALAM UPAYA REVITALISASI CRESCENTIANA EMY Akademi Farmasi Nasional PengaruhPEDAGANG Cara KAKI Pemanfaatan