3-Substituted Prolines: from Synthesis to Structural Applications, from Peptides to Foldamers †
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Al Exposure Increases Proline Levels by Different Pathways in An
www.nature.com/scientificreports OPEN Al exposure increases proline levels by diferent pathways in an Al‑sensitive and an Al‑tolerant rye genotype Alexandra de Sousa1,2, Hamada AbdElgawad2,4, Fernanda Fidalgo1, Jorge Teixeira1, Manuela Matos3, Badreldin A. Hamed4, Samy Selim5, Wael N. Hozzein6, Gerrit T. S. Beemster2 & Han Asard2* Aluminium (Al) toxicity limits crop productivity, particularly at low soil pH. Proline (Pro) plays a role in protecting plants against various abiotic stresses. Using the relatively Al‑tolerant cereal rye (Secale cereale L.), we evaluated Pro metabolism in roots and shoots of two genotypes difering in Al tolerance, var. RioDeva (sensitive) and var. Beira (tolerant). Most enzyme activities and metabolites of Pro biosynthesis were analysed. Al induced increases in Pro levels in each genotype, but the mechanisms were diferent and were also diferent between roots and shoots. The Al‑tolerant genotype accumulated highest Pro levels and this stronger increase was ascribed to simultaneous activation of the ornithine (Orn)‑biosynthetic pathway and decrease in Pro oxidation. The Orn pathway was particularly enhanced in roots. Nitrate reductase (NR) activity, N levels, and N/C ratios demonstrate that N‑metabolism is less inhibited in the Al‑tolerant line. The correlation between Pro changes and diferences in Al‑sensitivity between these two genotypes, supports a role for Pro in Al tolerance. Our results suggest that diferential responses in Pro biosynthesis may be linked to N‑availability. Understanding the role of Pro in diferences between genotypes in stress responses, could be valuable in plant selection and breeding for Al resistance. Proline (Pro) is involved in a wide range of plant physiological and developmental processes1. -

Flexible Fluidic Actuators for Soft Robotic Applications
University of Wollongong Research Online University of Wollongong Thesis Collection 2017+ University of Wollongong Thesis Collections 2019 Flexible Fluidic Actuators for Soft Robotic Applications Weiping Hu Follow this and additional works at: https://ro.uow.edu.au/theses1 University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Research Online is the open access institutional repository for the University of -

Environmentally Controlled Curvature of Single Collagen Proteins
Environmentally controlled curvature of single collagen proteins Naghmeh Rezaei, Aaron Lyons and Nancy R. Forde* Department of Physics Simon Fraser University 8888 University Drive Burnaby, BC V5A 1S6 CANADA *Corresponding author. 778-782-3161. [email protected]. ORCID: 0000-0002-5479-7073 - 1 - ABSTRACT The predominant structural protein in vertebrates is collagen, which plays a key role in extracellular matrix and connective tissue mechanics. Despite its prevalence and physical importance in biology, the mechanical properties of molecular collagen are far from established. The flexibility of its triple helix is unresolved, with descriptions from different experimental techniques ranging from flexible to semirigid. Furthermore, it is unknown how collagen type (homo- vs. heterotrimeric) and source (tissue-derived vs. recombinant) influence flexibility. Using SmarTrace, a chain tracing algorithm we devised, we performed statistical analysis of collagen conformations collected with atomic force microscopy (AFM) to Our results show that types I, II and III collagens the key fibrillar varieties exhibit molecular flexibilities that are very similar. However, collagen conformations are strongly modulated by salt, transitioning from compact to extended as KCl concentration increases, in both neutral and acidic pH. While analysis with a standard worm-like chain model suggests that the persistence length of collagen can attain almost any value within the literature range, closer inspection reveals that this modulation to changes in flexibility, but rather arises from the induction of curvature (either intrinsic or induced by interactions with the mica surface). By modifying standard polymer theory to include innate curvature, we show that collagen behaves as an equilibrated curved worm-like chain (cWLC) in two dimensions. -

Structural Insights Into Membrane Fusion Mediated by Convergent Small Fusogens
cells Review Structural Insights into Membrane Fusion Mediated by Convergent Small Fusogens Yiming Yang * and Nandini Nagarajan Margam Department of Microbiology and Immunology, Dalhousie University, Halifax, NS B3H 4R2, Canada; [email protected] * Correspondence: [email protected] Abstract: From lifeless viral particles to complex multicellular organisms, membrane fusion is inarguably the important fundamental biological phenomena. Sitting at the heart of membrane fusion are protein mediators known as fusogens. Despite the extensive functional and structural characterization of these proteins in recent years, scientists are still grappling with the fundamental mechanisms underlying membrane fusion. From an evolutionary perspective, fusogens follow divergent evolutionary principles in that they are functionally independent and do not share any sequence identity; however, they possess structural similarity, raising the possibility that membrane fusion is mediated by essential motifs ubiquitous to all. In this review, we particularly emphasize structural characteristics of small-molecular-weight fusogens in the hope of uncovering the most fundamental aspects mediating membrane–membrane interactions. By identifying and elucidating fusion-dependent functional domains, this review paves the way for future research exploring novel fusogens in health and disease. Keywords: fusogen; SNARE; FAST; atlastin; spanin; myomaker; myomerger; membrane fusion 1. Introduction Citation: Yang, Y.; Margam, N.N. Structural Insights into Membrane Membrane fusion -

Peptoid Residues Make Diverse, Hyperstable Collagen Triple Helices
Peptoid Residues Make Diverse, Hyperstable Collagen Triple Helices Julian L. Kessler1, Grace Kang1, Zhao Qin2, Helen Kang1, Frank G. Whitby3, Thomas E. Cheatham III4, Christopher P. Hill3, Yang Li1,*, and S. Michael Yu1,5 1Department of Biomedical Engineering, University of Utah, Salt Lake City, Utah 84112, USA 2Department of Civil & Environmental Engineering, Collagen of Engineering & Computer Science, Syracuse University, Syracuse, New York 13244, USA 3Department of Biochemistry, University of Utah School of Medicine, Salt Lake City, UT 84112, USA 4Department of Medicinal Chemistry, College of Pharmacy, L. S. Skaggs Pharmacy Research Institute, University of Utah, Salt Lake City, Utah 84112, USA 5Department of Pharmaceutics and Pharmaceutical Chemistry, University of Utah, Salt Lake City, Utah 84112, USA *Corresponding Author: Yang Li ([email protected]) Abstract The triple-helical structure of collagen, responsible for collagen’s remarkable biological and mechanical properties, has inspired both basic and applied research in synthetic peptide mimetics for decades. Since non-proline amino acids weaken the triple helix, the cyclic structure of proline has been considered necessary, and functional collagen mimetic peptides (CMPs) with diverse sidechains have been difficult to produce. Here we show that N-substituted glycines (N-glys), also known as peptoid residues, exhibit a general triple-helical propensity similar to or greater than proline, allowing synthesis of thermally stable triple-helical CMPs with unprecedented sidechain diversity. We found that the N-glys stabilize the triple helix by sterically promoting the preorganization of individual CMP chains into the polyproline-II helix conformation. Our findings were supported by the crystal structures of two atomic-resolution N-gly-containing CMPs, as well as experimental and computational studies spanning more than 30 N-gly-containing peptides. -

Low Proline Diet in Type I Hyperprolinaemia
Arch Dis Child: first published as 10.1136/adc.46.245.72 on 1 February 1971. Downloaded from Archives of Disease in Childhood, 1971, 46, 72. Low Proline Diet in Type I Hyperprolinaemia J. T. HARRIES, A. T. PIESOWICZ,* J. W. T. SEAKINS, D. E. M. FRANCIS, and 0. H. WOLFF From The Hospital for Sick Children, and the Institute of Child Health, University of London Harries, J. T., Piesowicz, A. T., Seakins, J. W. T., Francis, D. E. M., and Wolff, 0. H. (1971). Archives of Disease in Childhood, 46, 72. Low proline diet in type I hyperprolinaemia. A diagnosis of Type I hyperprolinaemia was made in a 7-month-old infant who presented with hypocalcaemic convulsions and malabsorp- tion. The plasma levels of proline were grossly raised and the urinary excretion of proline, hydroxyproline, and glycine was increased; neurological development was delayed and there were associated abnormalities of the electroencephalogram, renal tract, and bones. Restriction of dietary proline at the age of 9 months resulted in a prompt fall of plasma levels of proline to normal, and a low proline diet was continued until the age of 27 months when persistence of the biochemical defect was shown. During the period of dietary treatment, growth was satisfactory, mental development improved, and the electroencephalogram, and the renal, skeletal, and intestinal abnormalities disappeared. Proline should be regarded as a 'semi-essential' amino acid in the growing infant. Hyperprolinaemia, appearing in several members balance can be maintained on a proline-free diet copyright. of a family was first described by Scriver, Schafer, (Rose et al., 1955), and therefore the amino acid is and Efron in 1961. -
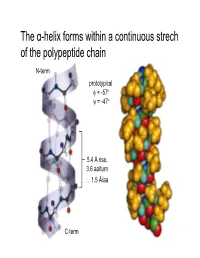
The Α-Helix Forms Within a Continuous Strech of the Polypeptide Chain
The α-helix forms within a continuous strech of the polypeptide chain N-term prototypical φ = -57 ° ψ = -47 ° 5.4 Å rise, 3.6 aa/turn ∴ 1.5 Å/aa C-term α-Helices have a dipole moment, due to unbonded and aligned N-H and C=O groups β-Sheets contain extended (β-strand) segments from separate regions of a protein prototypical φ = -139 °, ψ = +135 ° prototypical φ = -119 °, ψ = +113 ° (6.5Å repeat length in parallel sheet) Antiparallel β-sheets may be formed by closer regions of sequence than parallel Beta turn Figure 6-13 The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation • Interactions between adjacent R-groups – Ionic attraction or repulsion – Steric hindrance of adjacent bulky groups Helix wheel The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation • Interactions between adjacent R-groups – Ionic attraction or repulsion – Steric hindrance of adjacent bulky groups • Occurrence of proline and glycine • Interactions between ends of helix and aa R-groups His Glu N-term C-term -

Proline Oxidase Controls Proline, Glutamate, and Glutamine Cellular Concentrations in a U87 Glioblastoma Cell Line
RESEARCH ARTICLE Proline oxidase controls proline, glutamate, and glutamine cellular concentrations in a U87 glioblastoma cell line Pamela Cappelletti1,2*, Elena Tallarita1, Valentina Rabattoni1, Paola Campomenosi1,2, Silvia Sacchi1,2, Loredano Pollegioni1,2 1 Department of Biotechnology and Life Sciences, University of Insubria, Varese, Italy, 2 The Protein Factory Research Center, Politecnico of Milano and University of Insubria, Milano, Italy a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract L-Proline is a multifunctional amino acid that plays an essential role in primary metabolism and physiological functions. Proline is oxidized to glutamate in the mitochondria and the FAD-containing enzyme proline oxidase (PO) catalyzes the first step in L-proline degrada- OPEN ACCESS tion pathway. Alterations in proline metabolism have been described in various human dis- Citation: Cappelletti P, Tallarita E, Rabattoni V, eases, such as hyperprolinemia type I, velo-cardio-facial syndrome/Di George syndrome, Campomenosi P, Sacchi S, Pollegioni L (2018) schizophrenia and cancer. In particular, the mutation giving rise to the substitution Leu441- Proline oxidase controls proline, glutamate, and glutamine cellular concentrations in a U87 Pro was identified in patients suffering of schizophrenia and hyperprolinemia type I. Here, glioblastoma cell line. PLoS ONE 13(4): e0196283. we report on the expression of wild-type and L441P variants of human PO in a U87 glioblas- https://doi.org/10.1371/journal.pone.0196283 toma human cell line in an attempt to assess their effect on glutamate metabolism. The sub- Editor: Suzie Chen, Rutgers University, UNITED cellular localization of the flavoenzyme is not altered in the L441P variant, for which specific STATES activity is halved compared to the wild-type PO. -

The Fate of Arginine and Proline Carbon in Squid Tissuesl
Pacific Science (1982), vol. 36, no. 3 © 1983 by the University of Hawaii Press. All rights reserved The Fate of Arginine and Proline Carbon in Squid Tissuesl T. P. MOMMSEN,2 C. J. FRENCH,2 B. EMMETI,2 and P. W. HOCHACHKA2 ABSTRACT: The metabolism of proline and arginine was investigated in kidney, gill, and heart of the pelagic squid, Symplectoteuthis. The rates of CO2 release from 14C-proline exceeded the rates from 14C-arginine. The metabolic rate of arginine and proline was assessed by monitoring the incorporation of arginine-derived carbon into various intermediates. Arginine was metabolized, through ornithine, to proline as well as to glutamate and various subsequent derivatives (alanine, octopine, aspartate, and carboxylic acids). The same com ponents became labeled using 14C-proline as the starting substrate, but only the gill was capable ofconverting proline to arginine via the urea cycle. In addition, 14C-proline oxidation rates were high enough to exceed those of 14C-glucose in at least three tissues, kidney, heart, and inner mantle muscle. AT LEAST IN PART because ofthe large pool size data for heart, gill, and kidney from the squid, of free amino acids in cephalopod muscles Symplectoteuthis, showing the capacity for ar (e.g., see Hochachka, French, and Meredith ginine conversion to proline. The conversion 1978), interest recently has been focusing ofproline to arginine was measurable only in on their possible roles in energy metabolism. the gill. Although qualitatively similar to re During metabolic studies on the 1979 Alpha sults obtained with other species, these data Helix Cephalopod Expedition, relatively high also show some important, tissue-specific dif rates of CO2 release from arginine and ferences (Mommsen et al. -

Arginine Is Synthesized from Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates
0031-3998/11/6901-0046 Vol. 69, No. 1, 2011 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2010 International Pediatric Research Foundation, Inc. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates CHRIS TOMLINSON, MAHROUKH RAFII, MICHAEL SGRO, RONALD O. BALL, AND PAUL PENCHARZ Department of Paediatrics [C.T., M.S., P.P.], Research Institute [C.T., M.R., P.P.], The Hospital for Sick Children, Toronto, Ontario M5G1X8, Canada; Department of Nutritional Sciences [C.T., M.S., P.P.], University of Toronto, Toronto, Ontario M5S3E2, Canada; Department of Paediatrics [M.S.], St Michael’s Hospital, Toronto, Ontario M5B1W8, Canada; Department of Agricultural, Food and Nutritional Science [R.O.B., P.P.], University of Alberta, Edmonton, Alberta T6G2P5, Canada ABSTRACT: In neonatal mammals, arginine is synthesized in the litis (NEC) (8) and pulmonary hypertension (9). Furthermore, enterocyte, with either proline or glutamate as the dietary precursor. arginine supplementation was shown to reduce the incidence We have shown several times in piglets that proline is the only of all stages of NEC in moderately at risk infants (10) and a precursor to arginine, although in vitro evidence supports glutamate single bolus infusion of i.v. arginine improved oxygenation in in this role. Because of this uncertainty, we performed a multitracer infants with pulmonary hypertension (11). Therefore, because stable isotope study to determine whether proline, glutamate, or both are dietary precursors for arginine in enterally fed human neonates. arginine is clearly important for metabolism in the neonate, it Labeled arginine (M ϩ 2), proline (M ϩ 1), and glutamate (M ϩ 3) is critical to understand the metabolic pathways involved in its were given enterally to 15 stable, growing preterm infants (GA at synthesis. -

Y. Ma, X. Feng, J.A. Rogers, Y. Huang and Yihui
Lab on a Chip View Article Online CRITICAL REVIEW View Journal | View Issue Design and application of ‘J-shaped’ stress–strain Cite this: Lab Chip,2017,17,1689 behavior in stretchable electronics: a review Yinji Ma,ab Xue Feng,a John A. Rogers,c Yonggang Huangb and Yihui Zhang *a A variety of natural biological tissues (e.g., skin, ligaments, spider silk, blood vessel) exhibit ‘J-shaped’ stress–strain behavior, thereby combining soft, compliant mechanics and large levels of stretchability, with anatural‘strain-limiting’ mechanism to prevent damage from excessive strain. Synthetic materials with similar stress–strain behaviors have potential utility in many promising applications, such as tissue engineer- ing (to reproduce the nonlinear mechanical properties of real biological tissues) and biomedical devices (to enable natural, comfortable integration of stretchable electronics with biological tissues/organs). Recent advances in this field encompass developments of novel material/structure concepts, fabrication ap- proaches, and unique device applications. This review highlights five representative strategies, including de- signs that involve open network, wavy and wrinkled morphologies, helical layouts, kirigami and origami constructs, and textile formats. Discussions focus on the underlying ideas, the fabrication/assembly routes, Received 18th March 2017, and the microstructure–property relationships that are essential for optimization of the desired ‘J-shaped’ Accepted 21st April 2017 stress–strain responses. Demonstration applications provide examples of the use of these designs in de- formable electronics and biomedical devices that offer soft, compliant mechanics but with inherent robust- DOI: 10.1039/c7lc00289k ness against damage from excessive deformation. We conclude with some perspectives on challenges and rsc.li/loc opportunities for future research. -

Gifc-2018 Book of Abstracts
GIFC-2018 Giornate Italo-Francesi di Chimica Journées Franco-Italiennes de Chimie 16 – 18 April 2018 Grand Hotel Savoia Via Arsenale di Terra, 5 Genova BOOK OF ABSTRACTS ISBN: 978-88-94952-00-1 Gold Sponsors Silver Sponsors Patronages 2 3 Scientific Program 4 5 6 List of Posters (according to alphabetic order of presenting Authors) 1st session, Monday 16th April Num Cognome Nome Contatto Titolo Synthesis, purification and characterization of PO1 Aboudou Soioulata [email protected] antimicrobial peptides isolated from animal venoms [email protected] PO2 Ajmalghan Muthali Coverage dependent recombination mechanisms of hydrogen from niv-amu.fr tungsten surfaces via density functional theory Hybrid squaraine-silica nanoparticles as nir probes for biological PO3 Alberto Gabriele [email protected] applications: optimization of the photoemission performances Synthesis of doped metal oxide nanocrystals for solution-processed PO4 Alkarsifi Riva [email protected] interfacial layers in organic solar cells PO5 Anceschi Anastasia [email protected] Maltodextrins nanosponges as precursor for porous carbon materials [email protected] PO7 Arnodo Davide First racemic total synthesis of heliolactone .it [email protected]. PO8 Azzi Emanuele Synthesis of boronated analogue of curcumin as potential therapeutical it agents for alzheimer’s disease Study of Nanobiomaterials with Bio-based Antioxidants: Interaction of PO9 Barzan Giulia Sci piem Polyphenol Molecules with Hydroxyapatite and Silica Luigi Methotrexate loaded solid lipid nanoparticles: protein functionalization to PO10 Battaglia [email protected] Sebastiano improve brain biodistribution [email protected] PO11 Begni Federico On the adsorption of toluene on porous materials with different chemical m composition Synthesischaracterization of activated carbon from modified banana peels PO12 Ben Khalifa Eya [email protected] for hexavalent chromium adsorption [email protected] PO13 Benvenuti Martino The maturation of the co-dehydrogenase from thermococcus sp.