Calcium Acetate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How to Take Your Phosphate Binders
How to take your phosphate binders Information for renal patients Oxford Kidney Unit Page 2 What are phosphate binders? To reduce the amount of phosphate you absorb from your food you may have been prescribed a medicine called a phosphate binder. Phosphate binders work by binding (attaching) to some of the phosphate in food. This will reduce the amount of phosphate being absorbed into your blood stream. A list of phosphate binders and how to take them is shown below. Phosphate binder How to take it Calcichew (calcium carbonate) Chew thoroughly 10-15 minutes before or immediately before food Renacet (calcium acetate) Phosex (calcium acetate) Osvaren (calcium acetate and magnesium carbonate) Swallow whole after the first Renagel 2-3 mouthfuls of food (sevelemer hydrochloride) Renvela tablets (sevelemer carbonate) Alucaps (aluminium hydroxide) Renvela powder Dissolve in 60ml of water and (sevelemer carbonate) take after the first 2-3 mouthfuls of food Fosrenol tablets Chew thoroughly towards the (lanthanum carbonate) end/immediately after each meal Fosrenol powder Mix with a small amount of (lanthanum carbonate) food and eat immediately Velphoro Chew thoroughly after the first (sucroferric oxyhydroxide) 2-3 mouthfuls The phosphate binder you have been prescribed is: ……………………………………………………………………………………………………………………………………………………….. Page 3 How many phosphate binders should I take? You should follow the dose that has been prescribed for you. Your renal dietitian can advise how best to match your phosphate binders to your meal pattern, as well as which snacks require a phosphate binder. What happens if I forget to take my phosphate binder? For best results, phosphate binders should be taken as instructed. -

Calcium Acetate Capsules
Calcium Acetate Capsules Type of Posting Revision Bulletin Posting Date 27–Dec–2019 Official Date 01–Jan–2020 Expert Committee Chemical Medicines Monographs 6 Reason for Revision Compliance In accordance with the Rules and Procedures of the 2015–2020 Council of Experts, the Chemical Medicines Monographs 6 Expert Committee has revised the Calcium Acetate Capsules monograph. The purpose for the revision is to add Dissolution Test 4 to accommodate FDA-approved drug products with different dissolution conditions and/or tolerances than the existing dissolution tests. • Dissolution Test 4 was validated using a YMC-Pack ODS-A C18 brand of L1 column. The typical retention time for calcium acetate is about 4.3 min. The Calcium Acetate Capsules Revision Bulletin supersedes the currently official monograph. Should you have any questions, please contact Michael Chang, Senior Scientific Liaison (301-230-3217 or [email protected]). C236679-M11403-CHM62015, rev. 00 20191227 Revision Bulletin Calcium 1 Official January 1, 2020 Calcium Acetate Capsules PERFORMANCE TESTS DEFINITION Change to read: Calcium Acetate Capsules contain NLT 90.0% and NMT · DISSOLUTION á711ñ 110.0% of the labeled amount of calcium acetate Test 1 (C4H6CaO4). Medium: Water; 900 mL IDENTIFICATION Apparatus 2: 50 rpm, with sinkers · A. The retention time of the calcium peak of the Sample Time: 10 min solution corresponds to that of the Standard solution, as Mobile phase, Standard solution, Chromatographic obtained in the Assay. system, and System suitability: Proceed as directed in · B. IDENTIFICATION TESTSÐGENERAL á191ñ, Chemical the Assay. Identification Tests, Acetate Sample solution: Pass a portion of the solution under test Sample solution: 67 mg/mL of calcium acetate from through a suitable filter of 0.45-µm pore size. -

Production and Testing of Calcium Magnesium Acetate in Maine
77 Majesty's Stationery Office, London, England, River. Res. Note FPL-0229. Forest Service, U.S. 1948. Department of Agriculture, Madison, Wis., 1974. 14. M.S. Aggour and A. Ragab. Safety and Soundness 20. W.L. James. Effect of Temperature and Moisture of Submerged Timber Bridge PU.ing. FHWA/MD In Content on Internal Friction and Speed of Sound terim Report AW082-231-046. FHWA, U.S. Depart in Douglas Fir. Forest Product Journal, Vol. ment of Transportation, June 1982. 11, No. 9, 1961, pp. 383-390, 15. B.O. Orogbemi. Equipment for Determining the 21. A, Burmester. Relationship Between Sound Veloc Dynami c Modulus of Submerged Bridge Timber Pil ity and Morphological, Physical, and Mechani ·ing. Master's thesis. University of Maryland, cal Properties of Wood. Holz als Roh und Wer College Park, 1980. stoff, Vol. 23, No. 6, 1965, pp. 227-236 (in 16. T.L. Wilkinson. Strength Evaluation of Round German) • Timber Piles. Res. Note FPL-101. Forest Ser 22. c.c. Gerhards. Stress Wave Speed and MOE of vice, U.S. Department of Agriculture, Madison, Weetgum Ranging from 150 to 15 Percent MC. Wis., 1968. Forest Product Journal, Vol. 25, No. 4, 1975, 17. J, Bodig and B.A. Jayne. Mechanics of Wood and pp. 51-57. Wood Composites. Van Nostrand, New York, 1982. 18. R.M. Armstrong. Structural Properties of Timber Piles, Behavior of Deep Foundations. Report STP-670, ASTM, Philadelphia, 1979, pp. 118-152. 19. B.A, Bendtsen. Bending Strength and Stiffness Publication of this paper sponsored by Committee on of Bridge Piles After 85 Years in the Milwaukee Structures Maintenance. -

Phosphate Binders
Pharmacy Info Sheet Phosphate Binders calcium acetate, calcium carbonate (Tums, Calsan, Apocal, Ocal), calcium liquid, aluminum hydroxide (Basaljel, Amphojel), sevelamer (Renagel), lanthanum (Fosrenol) What it does: Phosphate binders are used to treat high Special considerations for lanthamum and blood phosphorus levels. sevelamer: Calcium acetate, calcium carbonate, calcium Lanthanum should be taken during or liquid, aluminum hydroxide, lanthanum and immediately after a meal. Taking a dose on an sevelamer bind dietary phosphate. When the empty stomach can cause nausea and kidneys fail, phosphorus builds up in the body vomiting. Chew the tablet completely before because the kidneys can no longer remove swallowing. DO NOT swallow tablets whole. much phosphorus. Phosphate binders are used to lower the amount of phosphorus Sevelamer should be taken just before eating. absorbed from food to limit development of Swallow the tablet whole – Renagel should not bone and blood vessel disease. be cut or chewed. The contents of sevelamer tablets expand in water and could cause Aluminum hydroxide and calcium carbonate choking if cut chewed or crushed. may also be prescribed as antacids. Calcium preparations may also be prescribed as Phosphate binders may interfere with the calcium supplements. Use them only as absorption of certain drugs such as iron prescribed. When these medications are supplements, antibiotics, digoxin, ranitidine, prescribed as calcium supplements or antiseizure, and antiarrhythmic medications. antacids, take between meals. If you are prescribed any of these drugs, take them at least 1 hour before or 3 hours after your How it works: phosphate binder. Kidney disease can cause phosphate to accumulate which results in bone and blood What to do if you miss a dose: vessel disease. -
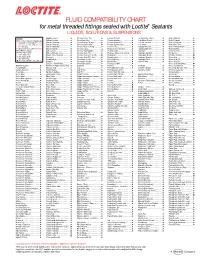
FLUID COMPATIBILITY CHART for Metal Threaded Fittings Sealed with Loctite¨ Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS
FLUID COMPATIBILITY CHART for metal threaded fittings sealed with Loctite® Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS LEGEND: Bagasse Fibers.......................... Chlorobenzene Dry ................... Ferrous Chloride ...................... Ion Exclusion Glycol ................. Nickel Chloride.......................... All Loctite® Anaerobic Sealants are Barium Acetate ........................ Chloroform Dry......................... Ferrous Oxalate......................... Irish Moss Slurry...................... Nickel Cyanide ......................... Compatible Including #242®, 243, Barium Carbonate..................... Chloroformate Methyl............... Ferrous Sulfate10%.................. Iron Ore Taconite ..................... Nickel Fluoborate ..................... 542, 545, 565, 567, 569, 571, 572, Barium Chloride........................ Chlorosulfonic Acid .................. Ferrous Sulfate (Sat)................. Iron Oxide ................................ Nickel Ore Fines ....................... 577, 580, 592 Barium Hydroxide..................... Chrome Acid Cleaning .............. Fertilizer Sol ............................. Isobutyl Alcohol ....................... Nickel Plating Bright ................. † Use Loctite® #270, 271™, 277, 554 Barium Sulfate.......................... Chrome Liquor.......................... Flotation Concentrates.............. Isobutyraldehyde ..................... Nickel Sulfate ........................... Not Recommended Battery Acid .............................. Chrome Plating -

Production of Low-Cost Acetate Deicers from Biomass and Industrial Wastes
Production of Low-Cost Acetate Deicers from Biomass and Industrial Wastes Shang-Tian Yang and Zuwei Jin, The Ohio State University Brian H. ChoUar, Federal Highway Administration Calcium magnesium acetate (CMA), a mixture of calcium 1 rom 10 million to 14 million tons of road salt are acetate and magnesium acetate, is used as an environmen• I used annually in the United States and Canada. tally benign roadway deicer. The present commercial F Salt is an extremely effective snow and ice con• CMA deicer made from glacial acetic acid and dolomitic trol agent and is relatively inexpensive. However, a lime or limestone is more expensive than salt and other study in New York State showed that although 1 ton of deicers. Also, a liquid potassium acetate deicer is used to road salt cost only $25, it caused more than $1,400 in replace urea and glycol in airport runway deicing. Two al• damage (1). Salt is corrosive to concrete and metals ternative low-cost methods to produce these acetate de- used in the nation's infrastructure, is harmful to road• icers from cheap feedstocks, such as biomass and side vegetation, and poses serious threats to environ• industrial wastes, were studied. CMA deicers produced ment and ground-water quality in some regions (2). from cheese whey by fermentation and extraction were FHWA spends about $12.5 billion annually, a sub• tested for their acetate content and deicing property. The stantial portion of which is used to rebuild and resur• CMA solid sample obtained from extraction of the acetic face highways and bridges damaged by salt corrosion. -

BLUE BOOK 1 Methyl Acetate CIR EXPERT PANEL MEETING
BLUE BOOK 1 Methyl Acetate CIR EXPERT PANEL MEETING AUGUST 30-31, 2010 Memorandum To: CIR Expert Panel Members and Liaisons From: Bart Heldreth Ph.D., Chemist Date: July 30, 2010 Subject: Draft Final Report of Methyl Acetate, Simple Alkyl Acetate Esters, Acetic Acid and its Salts as used in Cosmetics . This review includes Methyl Acetate and the following acetate esters, relevant metabolites and acetate salts: Propyl Acetate, Isopropyl Acetate, t-Butyl Acetate, Isobutyl Acetate, Butoxyethyl Acetate, Nonyl Acetate, Myristyl Acetate, Cetyl Acetate, Stearyl Acetate, Isostearyl Acetate, Acetic Acid, Sodium Acetate, Potassium Acetate, Magnesium Acetate, Calcium Acetate, Zinc Acetate, Propyl Alcohol, and Isopropyl Alcohol. At the June 2010 meeting, the Panel reviewed information submitted in response to an insufficient data announcement for HRIPT data for Cetyl Acetate at the highest concentration of use (lipstick). On reviewing the data in the report, evaluating the newly available unpublished studies and assessing the newly added ingredients, the Panel determined that the data are now sufficient, and issued a Tentative Report, with a safe as used conclusion. Included in this report are Research Institute for Fragrance Materials (RIFM) sponsored toxicity studies on Methyl Acetate and Propyl Acetate, which were provided in “wave 2” at the June Panel Meeting but are now incorporated in full. The Tentative Report was issued for a 60 day comment period (60 days as of the August panel meeting start date). The Panel should now review the Draft Final Report, confirm the conclusion of safe, and issue a Final Report. All of the materials are in the Panel book as well as in the URL for this meeting's web page http://www.cir- safety.org/aug10.shtml. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Calcium Acetate January 29, 2019
National Organic Standards Board Crops Subcommittee Petitioned Material Proposal Calcium Acetate January 29, 2019 Summary of Petition [Calcium Acetate]: Calcium acetate can occur naturally but is more often formulated by chelating finely ground limestone (calcium carbonate) with acetic acid. During this process calcium acetate is formed and comprises about 5% of the calcium in the final product. The remainder of the final product is primarily calcium carbonate. Other materials such as xantham gum and/or humic acids may be added to make a proprietary product. Calcium acetate has a variety of potential uses. This petition asks for approval for organic use as a soil amendment, plant micronutrient, soil pH adjuster and as a sunscald protectant. Calcium acetate is also currently registered for yellowjacket control in conventional crops. In each of these uses, the calcium acetate product is mixed with water and applied by spray to the crop, soil, or structure/covering. In the crops/soils use, the calcium acetate has an advantage in that it is much more water soluble than calcium carbonate and is more readily available to the plant. Other traditional sources of calcium, such as calcium carbonate, do not become water soluble until they have been acted on by soil microbes or acidic conditions. Products that include calcium acetate as well as other slower acting calcium sources can have both an immediate impact on the plant as well as an extended release effect as those less soluble materials are made plant available. For sunscald protection, the material acts to block direct transmission of sunlight due to its opacity. -

Influence of Calcium Acetate on Rye Bread Volume Katharina FUCKERER1*, Julia TREUDE1, Oliver HENSEL2, Joachim Johannes SCHMITT1
a Food Science and Technology ISSN 0101-2061 DDOI http://dx.doi.org/10.1590/1678-457X.03315 Influence of calcium acetate on rye bread volume Katharina FUCKERER1*, Julia TREUDE1, Dliver HENSEL2, Joachim Johannes SCHMITT1 Abstract The positive accepted savoury taste of rye bread is dependent on acetate concentration in the dough of such breads. In order to study how calcium acetate influences rye bread properties, the pH of rye doughs fortified with calcium acetate and the resulting volume of the breads were measured. Furthermore, CD2 formation of yeast with added calcium acetate and yeast with different pH levels (4, 7, 9) were measured. Thereby, it was determined that the addition of calcium acetate increased the pH of dough from 4.42 to 5.29 and significantly reduced the volume of the breads from 1235.19 mL to 885.52 mL. It could be proven that bread volume was affected by a 30.9% lower CD2 amount production of yeast, although bread volume was not affected by changing pH levels. Due to reduced bread volume, high concentrations of calcium acetate additions are not recommended for improving rye bread taste. Keywords: calcium acetate; volume; yeast; gas formation. Practical Application: Calcium acetate is not appropriate for improving rye bread taste. 1 Introduction Bread belongs to traditional food in Europe. Especially rye dough and gas formation of yeast in a rye flour and calcium bread with its typical savoury taste is favoured in Northern, acetate solution and a solution with different pH are measured. Central and Eastern Europe. This savoury taste is produced by adding sourdough to the dough, which contains yeast and lactic 2 Materials and methods acid bacteria (Birch et al., 2013; Gobbetti, 1998). -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

An Experimental and Numerical Model for the Release of Acetone from Decomposing EVA Containing Aluminium, Magnesium Or Calcium Hydroxide Fire Retardants
Article An experimental and numerical model for the release of acetone from decomposing EVA containing aluminium, magnesium or calcium hydroxide fire retardants Hewitt, Fiona, Rhebat, Diana Eid, Witkowski, Artur and Hull, T Richard Available at http://clok.uclan.ac.uk/16622/ Hewitt, Fiona, Rhebat, Diana Eid, Witkowski, Artur ORCID: 0000-0002-0005- 7520 and Hull, T Richard ORCID: 0000-0002-7970-4208 (2016) An experimental and numerical model for the release of acetone from decomposing EVA containing aluminium, magnesium or calcium hydroxide fire retardants. Polymer Degradation and Stability, 127 . pp. 65-78. ISSN 0141- 3910 It is advisable to refer to the publisher’s version if you intend to cite from the work. http://dx.doi.org/10.1016/j.polymdegradstab.2016.01.007 For more information about UCLan’s research in this area go to http://www.uclan.ac.uk/researchgroups/ and search for <name of research Group>. For information about Research generally at UCLan please go to http://www.uclan.ac.uk/research/ All outputs in CLoK are protected by Intellectual Property Rights law, including Copyright law. Copyright, IPR and Moral Rights for the works on this site are retained by the individual authors and/or other copyright owners. Terms and conditions for use of this material are defined in the policies page. CLoK Central Lancashire online Knowledge www.clok.uclan.ac.uk Fiona Hewitt, Diana Eid Rhebat, Artur Witkowski, T. Richard Hull, An experimental and numerical model for the release of acetone from decomposing EVA containing aluminium, magnesium or calcium hydroxide fire retardants, Polymer Degradation and Stability, Volume 127, 2016, Pages 65-78 An experimental and numerical model for the release of acetone from decomposing EVA containing aluminium, magnesium or calcium hydroxide fire retardants Authors: Fiona Hewitta, Diana Eid Rhebatb, Artur Witkowskia and T.