Clinical Protocol ZS-004
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How to Take Your Phosphate Binders
How to take your phosphate binders Information for renal patients Oxford Kidney Unit Page 2 What are phosphate binders? To reduce the amount of phosphate you absorb from your food you may have been prescribed a medicine called a phosphate binder. Phosphate binders work by binding (attaching) to some of the phosphate in food. This will reduce the amount of phosphate being absorbed into your blood stream. A list of phosphate binders and how to take them is shown below. Phosphate binder How to take it Calcichew (calcium carbonate) Chew thoroughly 10-15 minutes before or immediately before food Renacet (calcium acetate) Phosex (calcium acetate) Osvaren (calcium acetate and magnesium carbonate) Swallow whole after the first Renagel 2-3 mouthfuls of food (sevelemer hydrochloride) Renvela tablets (sevelemer carbonate) Alucaps (aluminium hydroxide) Renvela powder Dissolve in 60ml of water and (sevelemer carbonate) take after the first 2-3 mouthfuls of food Fosrenol tablets Chew thoroughly towards the (lanthanum carbonate) end/immediately after each meal Fosrenol powder Mix with a small amount of (lanthanum carbonate) food and eat immediately Velphoro Chew thoroughly after the first (sucroferric oxyhydroxide) 2-3 mouthfuls The phosphate binder you have been prescribed is: ……………………………………………………………………………………………………………………………………………………….. Page 3 How many phosphate binders should I take? You should follow the dose that has been prescribed for you. Your renal dietitian can advise how best to match your phosphate binders to your meal pattern, as well as which snacks require a phosphate binder. What happens if I forget to take my phosphate binder? For best results, phosphate binders should be taken as instructed. -

Calcium Acetate Capsules
Calcium Acetate Capsules Type of Posting Revision Bulletin Posting Date 27–Dec–2019 Official Date 01–Jan–2020 Expert Committee Chemical Medicines Monographs 6 Reason for Revision Compliance In accordance with the Rules and Procedures of the 2015–2020 Council of Experts, the Chemical Medicines Monographs 6 Expert Committee has revised the Calcium Acetate Capsules monograph. The purpose for the revision is to add Dissolution Test 4 to accommodate FDA-approved drug products with different dissolution conditions and/or tolerances than the existing dissolution tests. • Dissolution Test 4 was validated using a YMC-Pack ODS-A C18 brand of L1 column. The typical retention time for calcium acetate is about 4.3 min. The Calcium Acetate Capsules Revision Bulletin supersedes the currently official monograph. Should you have any questions, please contact Michael Chang, Senior Scientific Liaison (301-230-3217 or [email protected]). C236679-M11403-CHM62015, rev. 00 20191227 Revision Bulletin Calcium 1 Official January 1, 2020 Calcium Acetate Capsules PERFORMANCE TESTS DEFINITION Change to read: Calcium Acetate Capsules contain NLT 90.0% and NMT · DISSOLUTION á711ñ 110.0% of the labeled amount of calcium acetate Test 1 (C4H6CaO4). Medium: Water; 900 mL IDENTIFICATION Apparatus 2: 50 rpm, with sinkers · A. The retention time of the calcium peak of the Sample Time: 10 min solution corresponds to that of the Standard solution, as Mobile phase, Standard solution, Chromatographic obtained in the Assay. system, and System suitability: Proceed as directed in · B. IDENTIFICATION TESTSÐGENERAL á191ñ, Chemical the Assay. Identification Tests, Acetate Sample solution: Pass a portion of the solution under test Sample solution: 67 mg/mL of calcium acetate from through a suitable filter of 0.45-µm pore size. -

Effect of Lanthanum Carbonate and Calcium Acetate in the Treatment of Hyperphosphatemia in Patients of Chronic Kidney Disease P
Research Article Effect of lanthanum carbonate and calcium acetate in the treatment of hyperphosphatemia in patients of chronic kidney disease P. Thomas Scaria, Reneega Gangadhar1, Ramdas Pisharody2 ABSTRACT OObjectives:bjectives: The tolerability and efficacy of lanthanum carbonate has not been studied in the Indian population. This study was, therefore, undertaken to compare the efficacy and tolerability of lanthanum carbonate with calcium acetate in patients with stage 4 chronic kidney disease. Departments of Pharmacology and DDesign:esign: A randomized open label two group cross-over study. Therapeutics, Government Medical MMaterialsaterials aandnd MMethods:ethods: Following Institutional Ethics Committee approval and valid College, Thiruvananthapuram, consent, patients with stage 4 chronic kidney disease were randomized to receive either 1 Pharmacology, Government lanthanum carbonate 500 mg thrice daily or calcium acetate 667 mg thrice daily for Medical College, Kottayam, 4 weeks. After a 4-week washout period, the patients were crossed over for another 2Nephrology, Government Medical College, Thiruvananthapuram, 4 weeks. Serum phosphorous, serum calcium, serum alkaline phosphatase, and serum Kerala, India creatinine were estimated at fixed intervals. RResults:esults: Twenty-six patients were enrolled in the study. The mean serum phosphorous RReceived:eceived: 28.03.2008 concentrations showed a declining trend with lanthanum carbonate (from pre-drug levels of RRevised:evised: 12.07.2008 7.88 ± 1.52 mg/dL-7.14 ± 1.51 mg/dL) and calcium acetate (from pre-drug levels of 7.54 ± AAccepted:ccepted: 11.07.2009 1.39 mg/dL-6.51 ± 1.38 mg/dL). A statistically significant difference was seen when comparing the change in serum calcium produced by these drugs (P < 0.05). -

Fosrenol-Pm-En.Pdf
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION FOSRENOL® lanthanum carbonate hydrate Chewable tablets, 250 mg, 500 mg, 750 mg, and 1000 mg, oral Phosphate binder ATC code: V03A E03 Takeda Canada Inc. Date of Initial Approval: 22 Adelaide Street West, December 14, 2012 Suite 3800 Toronto Ontario M5H 4E3 Date of Revision: January 5, 2021 Submission Control No: 242595 FOSRENOL® and the FOSRENOL Logo are registered trademarks of Shire International Licensing BV, a Takeda company. Takeda® and the Takeda Logo are trademarks of Takeda Pharmaceutical Company Limited, used under license. FOSRENOL® Product Monograph Page 1 of 31 RECENT MAJOR LABEL CHANGES Not applicable TABLE OF CONTENTS RECENT MAJOR LABEL CHANGES ............................................................................ 2 TABLE OF CONTENTS .................................................................................................. 2 PART I: HEALTH PROFESSIONAL INFORMATION .................................................... 4 1 INDICATIONS ...................................................................................................... 4 1.1 Pediatrics ..................................................................................................... 4 1.2 Geriatrics ..................................................................................................... 4 2 CONTRAINDICATIONS ....................................................................................... 4 DOSAGE AND ADMINISTRATION .................................................................... -

Production and Testing of Calcium Magnesium Acetate in Maine
77 Majesty's Stationery Office, London, England, River. Res. Note FPL-0229. Forest Service, U.S. 1948. Department of Agriculture, Madison, Wis., 1974. 14. M.S. Aggour and A. Ragab. Safety and Soundness 20. W.L. James. Effect of Temperature and Moisture of Submerged Timber Bridge PU.ing. FHWA/MD In Content on Internal Friction and Speed of Sound terim Report AW082-231-046. FHWA, U.S. Depart in Douglas Fir. Forest Product Journal, Vol. ment of Transportation, June 1982. 11, No. 9, 1961, pp. 383-390, 15. B.O. Orogbemi. Equipment for Determining the 21. A, Burmester. Relationship Between Sound Veloc Dynami c Modulus of Submerged Bridge Timber Pil ity and Morphological, Physical, and Mechani ·ing. Master's thesis. University of Maryland, cal Properties of Wood. Holz als Roh und Wer College Park, 1980. stoff, Vol. 23, No. 6, 1965, pp. 227-236 (in 16. T.L. Wilkinson. Strength Evaluation of Round German) • Timber Piles. Res. Note FPL-101. Forest Ser 22. c.c. Gerhards. Stress Wave Speed and MOE of vice, U.S. Department of Agriculture, Madison, Weetgum Ranging from 150 to 15 Percent MC. Wis., 1968. Forest Product Journal, Vol. 25, No. 4, 1975, 17. J, Bodig and B.A. Jayne. Mechanics of Wood and pp. 51-57. Wood Composites. Van Nostrand, New York, 1982. 18. R.M. Armstrong. Structural Properties of Timber Piles, Behavior of Deep Foundations. Report STP-670, ASTM, Philadelphia, 1979, pp. 118-152. 19. B.A, Bendtsen. Bending Strength and Stiffness Publication of this paper sponsored by Committee on of Bridge Piles After 85 Years in the Milwaukee Structures Maintenance. -

Phosphate Binders
Pharmacy Info Sheet Phosphate Binders calcium acetate, calcium carbonate (Tums, Calsan, Apocal, Ocal), calcium liquid, aluminum hydroxide (Basaljel, Amphojel), sevelamer (Renagel), lanthanum (Fosrenol) What it does: Phosphate binders are used to treat high Special considerations for lanthamum and blood phosphorus levels. sevelamer: Calcium acetate, calcium carbonate, calcium Lanthanum should be taken during or liquid, aluminum hydroxide, lanthanum and immediately after a meal. Taking a dose on an sevelamer bind dietary phosphate. When the empty stomach can cause nausea and kidneys fail, phosphorus builds up in the body vomiting. Chew the tablet completely before because the kidneys can no longer remove swallowing. DO NOT swallow tablets whole. much phosphorus. Phosphate binders are used to lower the amount of phosphorus Sevelamer should be taken just before eating. absorbed from food to limit development of Swallow the tablet whole – Renagel should not bone and blood vessel disease. be cut or chewed. The contents of sevelamer tablets expand in water and could cause Aluminum hydroxide and calcium carbonate choking if cut chewed or crushed. may also be prescribed as antacids. Calcium preparations may also be prescribed as Phosphate binders may interfere with the calcium supplements. Use them only as absorption of certain drugs such as iron prescribed. When these medications are supplements, antibiotics, digoxin, ranitidine, prescribed as calcium supplements or antiseizure, and antiarrhythmic medications. antacids, take between meals. If you are prescribed any of these drugs, take them at least 1 hour before or 3 hours after your How it works: phosphate binder. Kidney disease can cause phosphate to accumulate which results in bone and blood What to do if you miss a dose: vessel disease. -

Bright Pleiotropic Properties in Renal Failure and Dialysis Patients
Open Access http://www.jparathyroid.com Journal of Journal of Parathyroid Disease 2012,2(2),75–77 Hypothesis Beyond the phosphate binding effect of sevelamer; bright pleiotropic properties in renal failure and dialysis patients Mahmoud Rafieian-Kopaei1, Saeed Mardani2, Hamid Nasri3*, Seyed Seifollah Beladi Mousavi4 hree main mechanisms are responsible for Implication for health policy/practice/research/ hyperphosphatemia, firstly an impaired renal medical education phosphate excretion due to acute or chronic In addition to its labeled property of serum phosphate Trenal insufficiency, secondly a massive acute phosphate reduction in hemodialysis individuals, sevelamer may load, and finally a primary increase in renal phosphate have further effects on factors influencing vascular reabsorption (1-3). Hyperphosphatemia in chronic renal endothelium, like inhibition of progression of vascular failure is related with increased cardiovascular mortality calcification, reduction of fibroblast growth factor 23, and morbidity. Lowering the phosphate load and abolishing of circulating inflammatory and oxidative maintaining serum phosphorus levels within the normal molecules, reducing of total and LDL cholesterol, value are important therapeutic aims to improve clinical uric acid, and uremic toxins. Also sevelamer may outcomes in chronic renal failure patients (1-3). Treatment reduce blood absorption of advanced glycation end comprises of lessening intestinal phosphate absorption products and endotoxins from the intestine. Hence, it is affordable to -

Efficacy and Safety of Sucroferric Oxyhydroxide Compared with Sevelamer
Efficacy and safety of sucroferric oxyhydroxide compared with sevelamer hydrochloride in Japanese haemodialysis patients with hyperphosphataemia: A randomised, open-label, multicentre, 12-week Phase III study Running title: Safety and efficacy of PA21 vs sevelamer Fumihiko Koiwa1, Keitaro Yokoyama2,, Masafumi Fukagawa3, Akira Terao4, Tadao Akizawa5 1Division of Nephrology, Department of Medicine, Showa University Fujigaoka Hospital, Yokohama, Japan 2Division of Nephrology and Hypertension, Department of Internal Medicine, The Jikei University School of Medicine, Tokyo, Japan 3Division of Nephrology, Endocrinology and Metabolism, Tokai University School of Medicine, Isehara, Japan 4Biostatistics, Faculty of Pharmaceutical Sciences, Josai University, Sakado, Japan 5Division of Nephrology, Department of Medicine, Showa University School of Medicine, Tokyo, Japan Corresponding author: Fumihiko Koiwa Division of Nephrology, Department of Medicine, Showa University Fujigaoka Hospital, 1-30 Fujigaoka, Aoba-ku, Yokohama 227-8501, Japan Tel.: +81-45-971-1151 Fax: +81-45-973-3010 Email: [email protected] This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/nep.12891 This article is protected by copyright. All rights reserved. Abstract Aim: We aimed to investigate the non-inferiority of PA21 (sucroferric oxyhydroxide) to sevelamer hydrochloride (sevelamer) in terms of efficacy and safety in Japanese haemodialysis patients with hyperphosphataemia. Methods: In this Phase III, open-label, multicentre study, 213 haemodialysis patients with hyperphosphataemia were randomised to PA21 or sevelamer treatment for 12 weeks. The primary outcome was adjusted serum phosphorus concentration at the end of treatment; the non-inferiority of PA21 was confirmed if the upper limit of the two-sided 95% confidence interval (CI) is ≤0.32 mmol/L. -
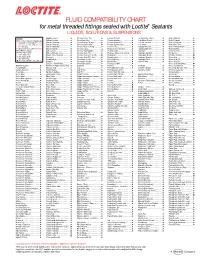
FLUID COMPATIBILITY CHART for Metal Threaded Fittings Sealed with Loctite¨ Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS
FLUID COMPATIBILITY CHART for metal threaded fittings sealed with Loctite® Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS LEGEND: Bagasse Fibers.......................... Chlorobenzene Dry ................... Ferrous Chloride ...................... Ion Exclusion Glycol ................. Nickel Chloride.......................... All Loctite® Anaerobic Sealants are Barium Acetate ........................ Chloroform Dry......................... Ferrous Oxalate......................... Irish Moss Slurry...................... Nickel Cyanide ......................... Compatible Including #242®, 243, Barium Carbonate..................... Chloroformate Methyl............... Ferrous Sulfate10%.................. Iron Ore Taconite ..................... Nickel Fluoborate ..................... 542, 545, 565, 567, 569, 571, 572, Barium Chloride........................ Chlorosulfonic Acid .................. Ferrous Sulfate (Sat)................. Iron Oxide ................................ Nickel Ore Fines ....................... 577, 580, 592 Barium Hydroxide..................... Chrome Acid Cleaning .............. Fertilizer Sol ............................. Isobutyl Alcohol ....................... Nickel Plating Bright ................. † Use Loctite® #270, 271™, 277, 554 Barium Sulfate.......................... Chrome Liquor.......................... Flotation Concentrates.............. Isobutyraldehyde ..................... Nickel Sulfate ........................... Not Recommended Battery Acid .............................. Chrome Plating -

Production of Low-Cost Acetate Deicers from Biomass and Industrial Wastes
Production of Low-Cost Acetate Deicers from Biomass and Industrial Wastes Shang-Tian Yang and Zuwei Jin, The Ohio State University Brian H. ChoUar, Federal Highway Administration Calcium magnesium acetate (CMA), a mixture of calcium 1 rom 10 million to 14 million tons of road salt are acetate and magnesium acetate, is used as an environmen• I used annually in the United States and Canada. tally benign roadway deicer. The present commercial F Salt is an extremely effective snow and ice con• CMA deicer made from glacial acetic acid and dolomitic trol agent and is relatively inexpensive. However, a lime or limestone is more expensive than salt and other study in New York State showed that although 1 ton of deicers. Also, a liquid potassium acetate deicer is used to road salt cost only $25, it caused more than $1,400 in replace urea and glycol in airport runway deicing. Two al• damage (1). Salt is corrosive to concrete and metals ternative low-cost methods to produce these acetate de- used in the nation's infrastructure, is harmful to road• icers from cheap feedstocks, such as biomass and side vegetation, and poses serious threats to environ• industrial wastes, were studied. CMA deicers produced ment and ground-water quality in some regions (2). from cheese whey by fermentation and extraction were FHWA spends about $12.5 billion annually, a sub• tested for their acetate content and deicing property. The stantial portion of which is used to rebuild and resur• CMA solid sample obtained from extraction of the acetic face highways and bridges damaged by salt corrosion. -

Aetna Formulary Exclusions Drug List
Covered and non-covered drugs Drugs not covered – and their covered alternatives 2020 Advanced Control Plan – Aetna Formulary Exclusions Drug List 05.03.525.1B (7/20) Below is a list of medications that will not be covered without a Key prior authorization for medical necessity. If you continue using one of these drugs without prior approval, you may be required UPPERCASE Brand-name medicine to pay the full cost. Ask your doctor to choose one of the generic lowercase italics Generic medicine or brand formulary options listed below. Preferred Options For Excluded Medications1 Excluded drug name(s) Preferred option(s) ABILIFY aripiprazole, clozapine, olanzapine, quetiapine, quetiapine ext-rel, risperidone, ziprasidone, VRAYLAR ABSORICA isotretinoin ACANYA adapalene, benzoyl peroxide, clindamycin gel (except NDC^ 68682046275), clindamycin solution, clindamycin-benzoyl peroxide, erythromycin solution, erythromycin-benzoyl peroxide, tretinoin, EPIDUO, ONEXTON, TAZORAC ACIPHEX, esomeprazole, lansoprazole, omeprazole, pantoprazole, DEXILANT ACIPHEX SPRINKLE ACTICLATE doxycycline hyclate capsule, doxycycline hyclate tablet (except doxycycline hyclate tablet 50 mg [NDC^ 72143021160 only], 75 mg, 150 mg), minocycline, tetracycline ACTOS pioglitazone ACUVAIL bromfenac, diclofenac, ketorolac, PROLENSA acyclovir cream acyclovir (except acyclovir cream), valacyclovir ADCIRCA sildenafil, tadalafil ADZENYS XR-ODT amphetamine-dextroamphetamine mixed salts ext-rel†, dexmethylphenidate ext-rel, dextroamphetamine ext-rel, methylphenidate ext-rel†, MYDAYIS, -

The Indirect Implication of SARS-Cov-2 Resulting in Kayexalate Toxicity in a Patient with Acute Kidney Injury
CODON P U B L I C A T I O N S Journal of Renal and Hepatic Disorders CASE REPORT: NEPHROLOGY The Indirect Implication of SARS-CoV-2 Resulting in Kayexalate Toxicity in a Patient with Acute Kidney Injury Charles E Middleton, IV, MD, William Daley, MD, Neha Varshney, MD Department of Pathology, University of Mississippi Medical Center, Jackson, MS, USA Abstract The clinical features of corona virus disease 2019 (COVID-19) are variable, but the majority of patients experience mild flu-like symptoms. The cases of severe disease include complications such as progressive pneumonia, acute kidney injury (AKI), multi-organ failure, and even death. This paper explores the association between COVID-19 and its effect on multiple organ systems and how the subsequent treatment of this dis- ease can itself lead to morbidity and mortality. We present a case that emphasizes the life-threatening gastrointestinal complications associated with the treatment of AKI in a patient with COVID-19. We conclude that the patients whose treatment regimens utilize medical resins should be closely monitored for gastrointestinal complications so as to mitigate the known adverse effects associated with these drugs, such as colonic mucosal ulceration, perforation, or even death. Keywords: acute kidney injury; colonic perforation; COVID-19; Kayexalate; resins; sevelamer Received: 25 November 2020; Accepted after revision: 5 February 2021; Published: 27 February 2021. Author for correspondence: Neha Varshney, MD, Assistant Professor, Department of Pathology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216-4500, USA. Email: [email protected] How to cite: Middleton CE, et al.