Phylogenetic Relationship Among Hylidae and Mitochondrial Protein-Coding Gene Expression in Response to Freezing and Ano
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downloaded from Brill.Com10/05/2021 09:34:25AM Via Free Access © Koninklijke Brill NV, Leiden, 2017
Amphibia-Reptilia 38 (2017): 483-502 Resurrection of genus Nidirana (Anura: Ranidae) and synonymizing N. caldwelli with N. adenopleura, with description of a new species from China Zhi-Tong Lyu, Zhao-Chi Zeng, Jian Wang, Chao-Yu Lin, Zu-Yao Liu, Ying-Yong Wang∗ Abstract. The taxonomy of Babina sensu lato was controversial in the past decades. In this study, the phylogeny of genus Babina sensu lato was re-constructed based on genetic analysis, morphological comparison and advertisement call analysis. We found that Babina sensu stricto and previous subgenus Nidirana should be two distinct genera in the family Ranidae. N. caldwelli is confirmed to be a synonym of N. adenopleura because of the small genetic divergence and the lack of distinct morphological differences. A new species, Nidirana nankunensis sp. nov. is described based on a series of specimens collected from Mt. Nankun, Guangdong Province, China, which can be distinguished from other known congeners by having a behavior of nest construction, distinctive advertisement calls, significant divergence in the mitochondrial genes, and a combination of morphological characters. Currently, the genus Babina contains two species and the genus Nidirana contains eight species. Keywords: Babina, bioacoustic, mitochondrial DNA, morphology, Nidirana nankunensis sp. nov., phylogeny. Introduction folds (Dubois, 1992). Subsequently, Nidirana was recognized as a separate genus by Chen The ranid genus Babina was established and de- et al. (2005), based on a molecular phyloge- scribed on the basis of Rana holsti Boulenger, netic tree of Southeast Asian ranids that only 1892 (type species) and Rana subaspera Bar- included one Nidirana species – R. (N.) cha- bour, 1908 by Thompson (1912). -

Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes Chrysoscelis
University of Dayton eCommons Honors Theses University Honors Program 4-1-2019 Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Maria P. LaBello University of Dayton Follow this and additional works at: https://ecommons.udayton.edu/uhp_theses Part of the Biology Commons eCommons Citation LaBello, Maria P., "Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis" (2019). Honors Theses. 218. https://ecommons.udayton.edu/uhp_theses/218 This Honors Thesis is brought to you for free and open access by the University Honors Program at eCommons. It has been accepted for inclusion in Honors Theses by an authorized administrator of eCommons. For more information, please contact [email protected], [email protected]. Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in Erythrocytes from the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Honors Thesis Maria P. LaBello Department: Biology Advisor: Carissa M. Krane, Ph.D. April 2019 Page | i Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Honors Thesis Maria P. LaBello Department: Biology Advisor: Carissa M. Krane, Ph.D. April 2019 Abstract Dryophytes chrysoscelis, commonly known as Cope’s gray treefrog, is a freeze tolerant anuran that freezes up to 65% of extracellular fluid during winter to survive. Glycerol is presumably used as a cryoprotectant during a period of cold-acclimation to protect cells from permanent damage due to hypoosmotic stress upon freezing and thawing. The passage of glycerol and water during cold-acclimation is mediated through aquaglyceroporin HC-3 in the nucleated erythrocytes (RBCs) of D. -
For Review Only
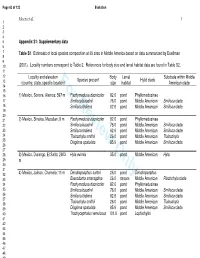
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Morphological Alterations in the External Gills of Some Tadpoles in Response to Ph
THIEME 142 Original Article Morphological Alterations in the External Gills of Some Tadpoles in Response to pH CaressaMaebhaThabah1 Longjam Merinda Devi1 Rupa Nylla Kynta Hooroo1 Sudip Dey2 1 Developmental Biology Laboratory, Department of Zoology, North- Address for correspondence Caressa Maebha Thabah, PhD in Eastern Hill University, Shillong, Meghalaya, India Zoology, Developmental Biology Laboratory, Department of Zoology, 2 Electron Microscope Laboratory, Sophisticated Analytical Instrument North-Eastern Hill University, Shillong 793022, Meghalaya, India Facility, North-Eastern Hill University, Shillong, Meghalaya, India (e-mail: [email protected]). J Morphol Sci 2018;35:142–152. Abstract Introduction Water pH affects the breeding, hatching, development, locomotion, mortality and habitat distributions of species in nature. The external gills of anuran tadpoles were studied by several authors in relation to abiotic factors. Exposure to low and high pH has been found to adversely affect the different tissues of various organisms. On that consideration, the present investigation was performed with tadpoles of the species Hyla annectans and Euphlyctis cyanophlyctis. Material and Methods The maximum and the minimum pH thresholds were determined prior to the detailed experiments on the effects of pH. The pH that demonstrated 50% mortality was taken as the minimum and maximum pH thresholds. The hatchlings of both the species were then subjected to different pH (based on the minimum and maximum pH thresholds). After 48 hours of exposure, the external gills of the hatchlings were anesthetized and observed under a scanning electron microscope. Keywords Results After 48 hours, clumping, overlapping and curling of the secondary filaments ► Hyla annectans of the external gills and epithelial lesions in response to both acidic and alkaline pH ► Euphlyctis were observed. -

Phylogeography of the American Green Treefrog Species Group
PHYLOGEOGRAPHY OF THE AMERICAN GREEN TREEFROG SPECIES GROUP by PAUL NATHANIEL PASICHNYK Presented to the Faculty of the Graduate School of The University of Texas at Arlington In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY THE UNIVERSITY OF TEXAS AT ARLINGTON November 2016 Copyright © by Paul N. Pasichnyk 2016 All Rights Reserved ii Acknowledgments A student’s graduate work is definitely not something that is done alone. I have received help from numerous people over the years and without their contributions, this would not have been possible. First, I would like to thank my parents for their tremendous support and motivation as I grew up and provided me with a foundation and love in education. Secondly, I would like to thank the person who has had to help me daily with tasks as simple as a pat on the back to as complex as reading a rough draft of a dissertation that might as well be in a foreign language, my wife Karen. My in-laws deserve special thanks as well, I do not believe that it is possible for others’ in-laws to be as supportive and helpful as mine. Then I would like to thank all the undergrads and friends who have helped see this to completion: Richard Hanna, Sari Mahon, Sarah Young, Nicole Lopez, Annamarie Slaven, Leslie Segovia, Pankaj BC, Matt Moseley, Christian Cox, Jeff Streicher, Claudia Marquez, Jesse Meik, Walter Schargel, Corey Roelke, Matt Watson, Rebbekah Watson, Thomas Eimermacher, Utpal Smart, David Sanchez, Jacobo Reyes-Velasco, Melissa Muenzler, Carl Franklin, Linda Taylor, Gloria Burlingham, Sherri Echols, and Paulette Williams. -

Short Toxin-Like Proteins Abound in Cnidaria Genomes
Toxins 2012, 4, 1367-1384; doi:10.3390/toxins4111367 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Article Short Toxin-like Proteins Abound in Cnidaria Genomes Yitshak Tirosh 1, Itai Linial 2, Manor Askenazi 1 and Michal Linial 1,* 1 Department of Biological Chemistry, Silberman Institute of Life Sciences, The Hebrew University of Jerusalem, Jerusalem 91904, Israel; E-Mails: [email protected] (Y.T.); [email protected] (M.A.) 2 The Racah Institute of Physics, The Hebrew University of Jerusalem, Jerusalem 91904, Israel; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +972-2-658-5425; Fax: +972-2-658-6448. Received: 24 September 2012; in revised form: 8 November 2012 / Accepted: 9 November 2012 / Published: 16 November 2012 Abstract: Cnidaria is a rich phylum that includes thousands of marine species. In this study, we focused on Anthozoa and Hydrozoa that are represented by the Nematostella vectensis (Sea anemone) and Hydra magnipapillata genomes. We present a method for ranking the toxin-like candidates from complete proteomes of Cnidaria. Toxin-like functions were revealed using ClanTox, a statistical machine-learning predictor trained on ion channel inhibitors from venomous animals. Fundamental features that were emphasized in training ClanTox include cysteines and their spacing along the sequences. Among the 83,000 proteins derived from Cnidaria representatives, we found 170 candidates that fulfill the properties of toxin-like-proteins, the vast majority of which were previously unrecognized as toxins. An additional 394 short proteins exhibit characteristics of toxin-like proteins at a moderate degree of confidence. -

Phylogeography Reveals an Ancient Cryptic Radiation in East-Asian Tree
Dufresnes et al. BMC Evolutionary Biology (2016) 16:253 DOI 10.1186/s12862-016-0814-x RESEARCH ARTICLE Open Access Phylogeography reveals an ancient cryptic radiation in East-Asian tree frogs (Hyla japonica group) and complex relationships between continental and island lineages Christophe Dufresnes1, Spartak N. Litvinchuk2, Amaël Borzée3,4, Yikweon Jang4, Jia-Tang Li5, Ikuo Miura6, Nicolas Perrin1 and Matthias Stöck7,8* Abstract Background: In contrast to the Western Palearctic and Nearctic biogeographic regions, the phylogeography of Eastern-Palearctic terrestrial vertebrates has received relatively little attention. In East Asia, tectonic events, along with Pleistocene climatic conditions, likely affected species distribution and diversity, especially through their impact on sea levels and the consequent opening and closing of land-bridges between Eurasia and the Japanese Archipelago. To better understand these effects, we sequenced mitochondrial and nuclear markers to determine phylogeographic patterns in East-Asian tree frogs, with a particular focus on the widespread H. japonica. Results: We document several cryptic lineages within the currently recognized H. japonica populations, including two main clades of Late Miocene divergence (~5 Mya). One occurs on the northeastern Japanese Archipelago (Honshu and Hokkaido) and the Russian Far-East islands (Kunashir and Sakhalin), and the second one inhabits the remaining range, comprising southwestern Japan, the Korean Peninsula, Transiberian China, Russia and Mongolia. Each clade further features strong allopatric Plio-Pleistocene subdivisions (~2-3 Mya), especially among continental and southwestern Japanese tree frog populations. Combined with paleo-climate-based distribution models, the molecular data allowed the identification of Pleistocene glacial refugia and continental routes of postglacial recolonization. Phylogenetic reconstructions further supported genetic homogeneity between the Korean H. -

2021Harcourtajmres
Bangor University MASTERS BY RESEARCH Interspecific Differences in Treefrog Response to Artificial Light at Night and Spectral Manipulation Harcourt, Alexander Award date: 2021 Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 Interspecific Differences in Treefrog Response to Artificial Light at Night and Spectral Manipulation Understanding the effect of artificial light at night (ALAN) on biodiversity is a key research topic of the 21st Century. Evidence suggests that LED lighting may be particularly disruptive due to strong short-wavelength emissions. Spectral manipulation of LED lighting to reduce these emissions may mitigate some disturbance, although further research is required to assess its value in comparison with other techniques. The impact of LED lighting has been documented for many species, however, amphibians remain relatively under-studied. Amphibians may be particularly sensitive to the effects of ALAN due to specialised vision adapted for low-light environments and reduced mobility. -

No Evidence for the 'Rate-Of-Living' Theory Across the Tetrapod Tree of Life
Received: 23 June 2019 | Revised: 30 December 2019 | Accepted: 7 January 2020 DOI: 10.1111/geb.13069 RESEARCH PAPER No evidence for the ‘rate-of-living’ theory across the tetrapod tree of life Gavin Stark1 | Daniel Pincheira-Donoso2 | Shai Meiri1,3 1School of Zoology, Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel Abstract 2School of Biological Sciences, Queen’s Aim: The ‘rate-of-living’ theory predicts that life expectancy is a negative function of University Belfast, Belfast, United Kingdom the rates at which organisms metabolize. According to this theory, factors that accel- 3The Steinhardt Museum of Natural History, Tel Aviv University, Tel Aviv, Israel erate metabolic rates, such as high body temperature and active foraging, lead to organismic ‘wear-out’. This process reduces life span through an accumulation of bio- Correspondence Gavin Stark, School of Zoology, Faculty of chemical errors and the build-up of toxic metabolic by-products. Although the rate- Life Sciences, Tel Aviv University, Tel Aviv, of-living theory is a keystone underlying our understanding of life-history trade-offs, 6997801, Israel. Email: [email protected] its validity has been recently questioned. The rate-of-living theory has never been tested on a global scale in a phylogenetic framework, or across both endotherms and Editor: Richard Field ectotherms. Here, we test several of its fundamental predictions across the tetrapod tree of life. Location: Global. Time period: Present. Major taxa studied: Land vertebrates. Methods: Using a dataset spanning the life span data of 4,100 land vertebrate spe- cies (2,214 endotherms, 1,886 ectotherms), we performed the most comprehensive test to date of the fundamental predictions underlying the rate-of-living theory. -

Translocation of an Endangered Endemic Korean Treefrog Dryophytes Suweonensis
A. Borzée, Y.-I. Kim, Y.-E. Kim & Y. Jang / Conservation Evidence (2018) 15, 6-11 Translocation of an endangered endemic Korean treefrog Dryophytes suweonensis Amaël Borzée1,2, Ye Inn Kim2, Ye Eun Kim2* & Yikweon Jang2,3*. 1 Laboratory of Behavioral Ecology and Evolution, School of Biological Sciences, Seoul National University, Seoul, 08826, Republic of Korea 2 Department of Life Sciences and Division of EcoScience, Ewha Womans University, Seoul, 03760, Republic of Korea 3 Interdisciplinary Program of EcoCreative, Ewha Womans University, Seoul, 03760, Republic of Korea SUMMARY Endangered species in heavily modified landscapes may be vulnerable to extinction if no conservation plan is implemented. The Suweon treefrog Dryophytes suweonensis is an endemic endangered species from the Korean Peninsula. In an attempt to conserve the species, a translocation plan was implemented in the city of Suwon. The receptor site was a specially modified island in a reservoir. Egg clutches were collected from four nearby sites, and were hatched and reared in a laboratory during 2015. One hundred and fifty froglets were released at the new site. In 2016, one year after the translocation, calling male D. suweonensis, and newly hatched tadpoles and juveniles were recorded. Juveniles were seen until the last week before hibernation in autumn 2016. However, only a single male was recorded calling in 2017. The population was consequently considered functionally extinct. Failure of the translocation most likely arose from mismanagement of the vegetation surrounding the wetlands, and the resulting inability of the site to fulfil the ecological requirements of the species. The project allowed the development of rearing protocols for the species, and defined its ecological requirements. -

Gekkotan Lizard Taxonomy
3% 5% 2% 4% 3% 5% H 2% 4% A M A D R Y 3% 5% A GEKKOTAN LIZARD TAXONOMY 2% 4% D ARNOLD G. KLUGE V O 3% 5% L 2% 4% 26 NO.1 3% 5% 2% 4% 3% 5% 2% 4% J A 3% 5% N 2% 4% U A R Y 3% 5% 2 2% 4% 0 0 1 VOL. 26 NO. 1 JANUARY, 2001 3% 5% 2% 4% INSTRUCTIONS TO CONTRIBUTORS Hamadryad publishes original papers dealing with, but not necessarily restricted to, the herpetology of Asia. Re- views of books and major papers are also published. Manuscripts should be only in English and submitted in triplicate (one original and two copies, along with three cop- ies of all tables and figures), printed or typewritten on one side of the paper. Manuscripts can also be submitted as email file attachments. Papers previously published or submitted for publication elsewhere should not be submitted. Final submissions of accepted papers on disks (IBM-compatible only) are desirable. For general style, contributors are requested to examine the current issue of Hamadryad. Authors with access to publication funds are requested to pay US$ 5 or equivalent per printed page of their papers to help defray production costs. Reprints cost Rs. 2.00 or 10 US cents per page inclusive of postage charges, and should be ordered at the time the paper is accepted. Major papers exceeding four pages (double spaced typescript) should contain the following headings: Title, name and address of author (but not titles and affiliations), Abstract, Key Words (five to 10 words), Introduction, Material and Methods, Results, Discussion, Acknowledgements, Literature Cited (only the references cited in the paper). -

Progress and Prospects for Studies on Chinese Amphibians
Asian Herpetological Research 2010, 1(2): 64-85 DOI: 10.3724/SP.J.1245.2010.00064 Progress and Prospects for Studies on Chinese Amphibians FEI Liang, YE Changyuan and JIANG Jianping* Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China Abstract This work summarizes the history and progress of the studies on Chinese amphibians since they first ap- peared in the Chinese literature. A wide range of research has been carried out, including the history of the definition of amphibians, faunal surveys, systematic research, ecological research, biochemical research (isozyme and other proteins or peptides, chromosomes, DNA), anatomical research, embryological research, phylogenetic and zoogeographical re- search, and many others such as ultrastructure of organs, crossbreeding test, regeneration of organs, abnormality survey, acoustics, fossils, sperm ultrastructure and parasites. In addition, the prospects for studies on Chinese amphibians in future are proposed in this paper. Keywords progress, prospect, faunal survey, systematics, amphibian, China 1. Introduction on amphibian research for at least 3000 years: toads (maybe Bufo gargarizans) were equated to ugliness and China is located in east Asia and covers a land area of wickedness in ‘The Book of Songs’ (-3000 years ago), approximately 9.6 million km2. Due to the vast territory but frog (鼃, 黽) had been inscribed on bones or tortoise occupied by this country, extremely different landforms, shell approximately 16th − 11th centuray B.C. (Guo et al., complex environments, and diverse climates and vegeta- 1999). “人鱼” (Mermen, now called the Chinese giant tion, China is very rich in amphibians, not only contai- salamander, Andrias davidianus), “活师” ( meaning tad- ning extremely numerous rare and endemic species, but poles), and “黾” (meaning frogs) were mentioned in ‘The also preserving a large number of relic species.