Inhibitory Effect of CPXV012 Peptide on Enveloped and Non-Enveloped Viruses of Different Families
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intracellular Trafficking of HBV Particles
cells Review Intracellular Trafficking of HBV Particles Bingfu Jiang 1 and Eberhard Hildt 1,2,* 1 Department of Virology, Paul-Ehrlich-Institut, D-63225 Langen, Germany; [email protected] 2 German Center for Infection Research (DZIF), TTU Hepatitis, Marburg-Gießen-Langen, D63225 Langen, Germany * Correspondence: [email protected]; Tel.: +49-61-0377-2140 Received: 12 August 2020; Accepted: 2 September 2020; Published: 2 September 2020 Abstract: The human hepatitis B virus (HBV), that is causative for more than 240 million cases of chronic liver inflammation (hepatitis), is an enveloped virus with a partially double-stranded DNA genome. After virion uptake by receptor-mediated endocytosis, the viral nucleocapsid is transported towards the nuclear pore complex. In the nuclear basket, the nucleocapsid disassembles. The viral genome that is covalently linked to the viral polymerase, which harbors a bipartite NLS, is imported into the nucleus. Here, the partially double-stranded DNA genome is converted in a minichromosome-like structure, the covalently closed circular DNA (cccDNA). The DNA virus HBV replicates via a pregenomic RNA (pgRNA)-intermediate that is reverse transcribed into DNA. HBV-infected cells release apart from the infectious viral parrticle two forms of non-infectious subviral particles (spheres and filaments), which are assembled by the surface proteins but lack any capsid and nucleic acid. In addition, naked capsids are released by HBV replicating cells. Infectious viral particles and filaments are released via multivesicular bodies; spheres are secreted by the classic constitutive secretory pathway. The release of naked capsids is still not fully understood, autophagosomal processes are discussed. This review describes intracellular trafficking pathways involved in virus entry, morphogenesis and release of (sub)viral particles. -

Discovery of a Highly Divergent Hepadnavirus in Shrews from China
Virology 531 (2019) 162–170 Contents lists available at ScienceDirect Virology journal homepage: www.elsevier.com/locate/virology Discovery of a highly divergent hepadnavirus in shrews from China T Fang-Yuan Niea,b,1, Jun-Hua Tianc,1, Xian-Dan Lind,1, Bin Yuc, Jian-Guang Xinge, Jian-Hai Caof, ⁎ ⁎⁎ Edward C. Holmesg,h, Runlin Z. Maa, , Yong-Zhen Zhangb,h, a State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China b State Key Laboratory for Infectious Disease Prevention and Control; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases; Department of Zoonoses, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Changping, Beijing, China c Wuhan Center for Disease Control and Prevention, Wuhan, China d Wenzhou Center for Disease Control and Prevention, Wenzhou, Zhejiang Province, China e Wencheng Center for Disease Control and Prevention, Wencheng, Zhejiang Province, China f Longwan Center for Disease Control and Prevention, Longwan District, Wenzhou, Zhejiang Province, China g Marie Bashir Institute for Infectious Diseases and Biosecurity, Charles Perkins Centre, School of Life and Environmental Sciences and Sydney Medical School, The University of Sydney, Sydney, NSW 2006, Australia h Shanghai Public Health Clinical Center & Institute of Biomedical Sciences, Fudan University, Shanghai, China ARTICLE INFO ABSTRACT Keywords: Limited sampling means that relatively little is known about the diversity and evolutionary history of mam- Hepadnaviruses malian members of the Hepadnaviridae (genus Orthohepadnavirus). An important case in point are shrews, the Shrews fourth largest group of mammals, but for which there is limited knowledge on the role they play in viral evo- Phylogeny lution and emergence. -

Summary of Antimicrobial Activity
SUMMARY OF ANTIMICROBIAL ACTIVITY 3x RENEGADE DAILY ONE-STEP DISINFECTANT Description 3x RENEGADE DAILY Disinfectant & Detergent is a broad spectrum, hard surface disinfectant. When used as directed, this product will deliver effective biocidal action against bacteria, fungi, and viruses. This formulation is a blend of a premium active ingredients and inerts: surfactants, chelates, and water. Biocidal performance is attained when this product is properly diluted at 1/2 oz. per gallon or 1:256 (1 oz. per gallon or 1:128 for Norovirus). 3x RENEGADE DAILY can be used to disinfect a wide variety of hard surfaces such as floors, walls, toilets, sinks, and countertops in hospitals, households, and institutions. Regulatory Summary Physical Properties EPA Registration No. 6836-349- pH of Concentrate 12.0 – 13.5 Flash Point (PMCC) >200 F 12120 USDA Authorization None Specific Gravity @ 0.98 – 1.05 g/mL % Quat (mol. wt.342.0) 22.24 25°C California Status Pounds per gallon @ 8.42 – 8.51 % Volatile 93.5-94.5 25°C Canadian PCP# None Canadian Din # None Summary of Antimicrobial Test Results 3x RENEGADE DAILY is a "One-Step" Hospital Disinfectant, Virucide, Fungicide, Mildewstat, Sanitizer and Cleaner. Listed in the following pages is a summary of Antimicrobial Claims and a review of test results. Claim: Contact time: Organic Soil: Water Conditions: Disinfectant Varies 5% 250ppm as CaCO3 Test Method: AOAC Germicidal Spray Test Organism Contact Dilution Time (Min) 868 ppm (1/2oz. per Acinetobacter baumannii 3 Gal) Bordetella bronchiseptica 3 868 ppm Bordetella pertussis 3 868 ppm Campylobacter jejuni 3 868 ppm Enterobacter aerogenes 3 1736 PPM (1 oz per Gal) Enterococcus faecalis 3 868 ppm Enterococcus faecalis - Vancomycin resistant [VRE] 3 868 ppm Escherichia coli 3 868 ppm Escherichia coli [O157:H7] 3 868 ppm Escherichia coli ESBL – Extended spectrum beta- 868 ppm 10 lactamase containing E. -

Risk Groups: Viruses (C) 1988, American Biological Safety Association
Rev.: 1.0 Risk Groups: Viruses (c) 1988, American Biological Safety Association BL RG RG RG RG RG LCDC-96 Belgium-97 ID Name Viral group Comments BMBL-93 CDC NIH rDNA-97 EU-96 Australia-95 HP AP (Canada) Annex VIII Flaviviridae/ Flavivirus (Grp 2 Absettarov, TBE 4 4 4 implied 3 3 4 + B Arbovirus) Acute haemorrhagic taxonomy 2, Enterovirus 3 conjunctivitis virus Picornaviridae 2 + different 70 (AHC) Adenovirus 4 Adenoviridae 2 2 (incl animal) 2 2 + (human,all types) 5 Aino X-Arboviruses 6 Akabane X-Arboviruses 7 Alastrim Poxviridae Restricted 4 4, Foot-and- 8 Aphthovirus Picornaviridae 2 mouth disease + viruses 9 Araguari X-Arboviruses (feces of children 10 Astroviridae Astroviridae 2 2 + + and lambs) Avian leukosis virus 11 Viral vector/Animal retrovirus 1 3 (wild strain) + (ALV) 3, (Rous 12 Avian sarcoma virus Viral vector/Animal retrovirus 1 sarcoma virus, + RSV wild strain) 13 Baculovirus Viral vector/Animal virus 1 + Togaviridae/ Alphavirus (Grp 14 Barmah Forest 2 A Arbovirus) 15 Batama X-Arboviruses 16 Batken X-Arboviruses Togaviridae/ Alphavirus (Grp 17 Bebaru virus 2 2 2 2 + A Arbovirus) 18 Bhanja X-Arboviruses 19 Bimbo X-Arboviruses Blood-borne hepatitis 20 viruses not yet Unclassified viruses 2 implied 2 implied 3 (**)D 3 + identified 21 Bluetongue X-Arboviruses 22 Bobaya X-Arboviruses 23 Bobia X-Arboviruses Bovine 24 immunodeficiency Viral vector/Animal retrovirus 3 (wild strain) + virus (BIV) 3, Bovine Bovine leukemia 25 Viral vector/Animal retrovirus 1 lymphosarcoma + virus (BLV) virus wild strain Bovine papilloma Papovavirus/ -

Arenaviridae Astroviridae Filoviridae Flaviviridae Hantaviridae
Hantaviridae 0.7 Filoviridae 0.6 Picornaviridae 0.3 Wenling red spikefish hantavirus Rhinovirus C Ahab virus * Possum enterovirus * Aronnax virus * * Wenling minipizza batfish hantavirus Wenling filefish filovirus Norway rat hunnivirus * Wenling yellow goosefish hantavirus Starbuck virus * * Porcine teschovirus European mole nova virus Human Marburg marburgvirus Mosavirus Asturias virus * * * Tortoise picornavirus Egyptian fruit bat Marburg marburgvirus Banded bullfrog picornavirus * Spanish mole uluguru virus Human Sudan ebolavirus * Black spectacled toad picornavirus * Kilimanjaro virus * * * Crab-eating macaque reston ebolavirus Equine rhinitis A virus Imjin virus * Foot and mouth disease virus Dode virus * Angolan free-tailed bat bombali ebolavirus * * Human cosavirus E Seoul orthohantavirus Little free-tailed bat bombali ebolavirus * African bat icavirus A Tigray hantavirus Human Zaire ebolavirus * Saffold virus * Human choclo virus *Little collared fruit bat ebolavirus Peleg virus * Eastern red scorpionfish picornavirus * Reed vole hantavirus Human bundibugyo ebolavirus * * Isla vista hantavirus * Seal picornavirus Human Tai forest ebolavirus Chicken orivirus Paramyxoviridae 0.4 * Duck picornavirus Hepadnaviridae 0.4 Bildad virus Ned virus Tiger rockfish hepatitis B virus Western African lungfish picornavirus * Pacific spadenose shark paramyxovirus * European eel hepatitis B virus Bluegill picornavirus Nemo virus * Carp picornavirus * African cichlid hepatitis B virus Triplecross lizardfish paramyxovirus * * Fathead minnow picornavirus -

The Poxviridae Comprise a Family of Complex Cytoplasmic Replicating
The Poxviridae comprise a family of complex cytoplasmic replicating DNA viruses that includes a number of important human and animal pathogens such as Variola virus, the causative agent of smallpox, Monkeypox virus, Cowpox virus and Vaccinia virus. Despite the successfully eradication of smallpox as a human disease by the worldwide vaccination campaigns 30 years ago, there nowadays still remains a considerable fear that Variola virus could be used as a bioweapon. Additionally, the re-emergence of zoonotic poxviruses such as the human Monkeypox virus outbreak that occurred in 2003 in the United States, Cowpox virus outbreaks in Europe and Vaccinia virus outbreaks in Brazil and India in the last years renewed the interest in the search for new anti- poxviral drugs. Besides, there is only one antiviral agent currently approved by FDA (Food and Drug Administration) for use against orthopoxviruses: cidofovir. However there are some concerns about its use, since cidofovir therapy can lead to renal and ocular toxicity, and also because cidofovir-resistant strains of camelpox, cowpox, monkeypox and vaccinia viruses have been isolated. In this context, the two poxvirus proteases, I7 and G1, arise as interesting protein targets for anti-poxvirus drugs development. In fact, it was previously showed that both I7 and G1 proteolysis activity are essential for viral replication and that these proteins have low identity with cellular proteins. These facts highlighted them as attractive antiviral targets. However, much essential knowledge of the molecular mechanisms of both proteases remains unexplored. This project aims to investigate more deeply both the structure and the function of these proteases. -

Mechanism of Hepatitis B Virus Cccdna Formation
viruses Review Mechanism of Hepatitis B Virus cccDNA Formation Lei Wei and Alexander Ploss * 110 Lewis Thomas Laboratory, Department of Molecular Biology, Princeton University, Washington Road, Princeton, NJ 08544, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-609-258-7128 Abstract: Hepatitis B virus (HBV) remains a major medical problem affecting at least 257 million chronically infected patients who are at risk of developing serious, frequently fatal liver diseases. HBV is a small, partially double-stranded DNA virus that goes through an intricate replication cycle in its native cellular environment: human hepatocytes. A critical step in the viral life-cycle is the conversion of relaxed circular DNA (rcDNA) into covalently closed circular DNA (cccDNA), the latter being the major template for HBV gene transcription. For this conversion, HBV relies on multiple host factors, as enzymes capable of catalyzing the relevant reactions are not encoded in the viral genome. Combinations of genetic and biochemical approaches have produced findings that provide a more holistic picture of the complex mechanism of HBV cccDNA formation. Here, we review some of these studies that have helped to provide a comprehensive picture of rcDNA to cccDNA conversion. Mechanistic insights into this critical step for HBV persistence hold the key for devising new therapies that will lead not only to viral suppression but to a cure. Keywords: hepatitis B virus; HBV; viral replication; cccDNA biogenesis; rcDNA; DNA repair Citation: Wei, L.; Ploss, A. 1. Overview of HBV Life Cycle and cccDNA Biogenesis Mechanism of Hepatitis B Virus cccDNA Formation. Viruses 2021, 13, The hepatotropic HBV belongs to the Hepadnaviridae family and is a blood-borne 1463. -

Here, There, and Everywhere: the Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses
viruses Review Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses Natalia Ingrid Oliveira Silva, Jaqueline Silva de Oliveira, Erna Geessien Kroon , Giliane de Souza Trindade and Betânia Paiva Drumond * Laboratório de Vírus, Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais: Belo Horizonte, Minas Gerais 31270-901, Brazil; [email protected] (N.I.O.S.); [email protected] (J.S.d.O.); [email protected] (E.G.K.); [email protected] (G.d.S.T.) * Correspondence: [email protected] Abstract: The global emergence of zoonotic viruses, including poxviruses, poses one of the greatest threats to human and animal health. Forty years after the eradication of smallpox, emerging zoonotic orthopoxviruses, such as monkeypox, cowpox, and vaccinia viruses continue to infect humans as well as wild and domestic animals. Currently, the geographical distribution of poxviruses in a broad range of hosts worldwide raises concerns regarding the possibility of outbreaks or viral dissemination to new geographical regions. Here, we review the global host ranges and current epidemiological understanding of zoonotic orthopoxviruses while focusing on orthopoxviruses with epidemic potential, including monkeypox, cowpox, and vaccinia viruses. Keywords: Orthopoxvirus; Poxviridae; zoonosis; Monkeypox virus; Cowpox virus; Vaccinia virus; host range; wild and domestic animals; emergent viruses; outbreak Citation: Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range 1. Poxvirus and Emerging Diseases and Geographic Distribution of Zoonotic diseases, defined as diseases or infections that are naturally transmissible Zoonotic Orthopoxviruses. Viruses from vertebrate animals to humans, represent a significant threat to global health [1]. -

Downloaded from Transcriptome Shotgun Assembly (TSA) Database on 29 November 2020 (Ftp://Ftp.Ddbj.Nig.Ac.Jp/Ddbj Database/Tsa/, Table S3)
viruses Article Discovery and Characterization of Actively Replicating DNA and Retro-Transcribing Viruses in Lower Vertebrate Hosts Based on RNA Sequencing Xin-Xin Chen, Wei-Chen Wu and Mang Shi * School of Medicine, Sun Yat-sen University, Shenzhen 518107, China; [email protected] (X.-X.C.); [email protected] (W.-C.W.) * Correspondence: [email protected] Abstract: In a previous study, a metatranscriptomics survey of RNA viruses in several important lower vertebrate host groups revealed huge viral diversity, transforming the understanding of the evolution of vertebrate-associated RNA virus groups. However, the diversity of the DNA and retro-transcribing viruses in these host groups was left uncharacterized. Given that RNA sequencing is capable of revealing viruses undergoing active transcription and replication, we collected previously generated datasets associated with lower vertebrate hosts, and searched them for DNA and retro-transcribing viruses. Our results revealed the complete genome, or “core gene sets”, of 18 vertebrate-associated DNA and retro-transcribing viruses in cartilaginous fishes, ray- finned fishes, and amphibians, many of which had high abundance levels, and some of which showed systemic infections in multiple organs, suggesting active transcription or acute infection within the host. Furthermore, these new findings recharacterized the evolutionary history in the families Hepadnaviridae, Papillomaviridae, and Alloherpesviridae, confirming long-term virus–host codivergence relationships for these virus groups. -

New Avian Hepadnavirus in Palaeognathous Bird, Germany
RESEARCH LETTERS New Avian Hepadnavirus includes emus (Dromaius novaehollandiae) and ostrich- es (Struthio spp.). in Palaeognathous Bird, In 2015, a deceased adult elegant-crested tinamou Germany kept at Wuppertal Zoo (Wuppertal, Germany) underwent necropsy at the University of Veterinary Medicine Han- 1 1 nover, Foundation (Hannover, Germany). Initial histologic Wendy K. Jo, Vanessa M. Pfankuche, examination revealed moderate, necrotizing hepatitis and Henning Petersen, Samuel Frei, Maya Kummrow, inclusion body–like structures within the hepatocytes. To Stephan Lorenzen, Martin Ludlow, Julia Metzger, identify a putative causative agent, we isolated nucleic ac- Wolfgang Baumgärtner, Albert Osterhaus, ids from the liver and prepared them for sequencing on an Erhard van der Vries Illumina MiSeq system (Illumina, San Diego, CA, USA) Author affiliations: University of Veterinary Medicine Hannover, (online Technical Appendix, https://wwwnc.cdc.gov/EID/ Foundation, Hannover, Germany (W.K. Jo, V.M. Pfankuche, article/23/12/16-1634-Techapp1.pdf). We compared ob- H. Petersen, M. Ludlow, J. Metzger, W. Baumgärtner, tained reads with sequences in GenBank using an in-house A. Osterhaus, E. van der Vries); Center for Systems metagenomics pipeline. Approximately 78% of the reads Neuroscience, Hannover (W.K. Jo, V.M. Pfankuche, aligned to existing avihepadnavirus sequences. A full ge- W. Baumgärtner, A. Osterhaus); Wuppertal Zoo, Wuppertal, nome (3,024 bp) of the putative elegant-crested tinamou Germany (S. Frei, M. Kummrow); Bernhard Nocht Institute for HBV (ETHBV) was subsequently constructed by de novo Tropical Medicine, Hamburg (S. Lorenzen); Artemis One Health, assembly mapping >2 million reads (88.6%) to the virus Utrecht, the Netherlands (A. Osterhaus) genome (GenBank accession no. -
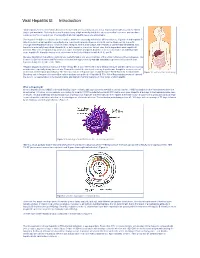
Viral Hepatitis B: Introduction
Viral Hepatitis B: Introduction “Viral hepatitis," refers to infections that affect the liver and are caused by viruses. It is a major public health issue in the United States and worldwide. Not only does viral hepatitis carry a high morbidity, but it also stresses medical resources and can have severe economic consequences. The majority of all viral hepatitis cases are preventable. Viral hepatitis includes five distinct disease entities, which are caused by at least five different viruses. Hepatitis A and hepatitis B (infectious and serum hepatitis, respectively) are considered separate diseases and both can be diagnosed by a specific serologic test. Hepatitis C and E comprise a third category, each a distinct type, with Hepatitis C parenterally transmitted, and hepatitis E enterically transmitted. Hepatitis D, or delta hepatitis, is another distinct virus that is dependent upon hepatitis B infection. This form of hepatitis may occur as a super-infectionin a hepatitis B carrier or as a co-infection in an individual with acute hepatitis B. Hepatitis viruses most often found in the United States include A, B, C, and D. Because fatality from hepatitis is relatively low, mortality figures are a poor indicator of the actual incidence of these diseases. The Centers for Disease Control and Prevention estimated that approximately 400,000–600,000 people were infected with viral hepatitis during the decade of the 1990s. Hepatitis plagued mankind as early as the fifth century BC. It was referenced in early biblical literature and described as occurring in outbreaks, especially during times of war. Toward the end of the nineteenth century, hepatitis was thought to occur as a result of infection of the hepatic parenchyma. -

Conventional and Unconventional Mechanisms for Capping Viral Mrna
REVIEWS Conventional and unconventional mechanisms for capping viral mRNA Etienne Decroly1, François Ferron1, Julien Lescar1,2 and Bruno Canard1 Abstract | In the eukaryotic cell, capping of mRNA 5′ ends is an essential structural modification that allows efficient mRNA translation, directs pre-mRNA splicing and mRNA export from the nucleus, limits mRNA degradation by cellular 5′–3′ exonucleases and allows recognition of foreign RNAs (including viral transcripts) as ‘non-self’. However, viruses have evolved mechanisms to protect their RNA 5′ ends with either a covalently attached peptide or a cap moiety (7‑methyl-Gppp, in which p is a phosphate group) that is indistinguishable from cellular mRNA cap structures. Viral RNA caps can be stolen from cellular mRNAs or synthesized using either a host- or virus-encoded capping apparatus, and these capping assemblies exhibit a wide diversity in organization, structure and mechanism. Here, we review the strategies used by viruses of eukaryotic cells to produce functional mRNA 5′-caps and escape innate immunity. Pre-mRNA splicing The cap structure found at the 5′ end of eukaryotic reactions involved in the viral RNA-capping process, A post-transcriptional mRNAs consists of a 7‑methylguanosine (m7G) moi‑ and the specific cellular factors that trigger a response modification of pre-mRNA, in ety linked to the first nucleotide of the transcript via a from the innate immune system. which introns are excised and 5′–5′ triphosphate bridge1 (FIG. 1a). The cap has several exons are joined in order to form a translationally important biological roles, such as protecting mRNA Capping, decapping and turnover of host RNA functional, mature mRNA.