Summary of Antimicrobial Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: a Review
viruses Review Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review Dong Joo Seo 1 and Changsun Choi 2,* 1 Department of Food Science and Nutrition, College of Health and Welfare and Education, Gwangju University 277 Hyodeok-ro, Nam-gu, Gwangju 61743, Korea; [email protected] 2 Department of Food and Nutrition, School of Food Science and Technology, College of Biotechnology and Natural Resources, Chung-Ang University, 4726 Seodongdaero, Daeduck-myun, Anseong-si, Gyeonggi-do 17546, Korea * Correspondence: [email protected]; Tel.: +82-31-670-4589; Fax: +82-31-676-8741 Abstract: Mushrooms are used in their natural form as a food supplement and food additive. In addition, several bioactive compounds beneficial for human health have been derived from mushrooms. Among them, polysaccharides, carbohydrate-binding protein, peptides, proteins, enzymes, polyphenols, triterpenes, triterpenoids, and several other compounds exert antiviral activity against DNA and RNA viruses. Their antiviral targets were mostly virus entry, viral genome replication, viral proteins, and cellular proteins and influenced immune modulation, which was evaluated through pre-, simultaneous-, co-, and post-treatment in vitro and in vivo studies. In particular, they treated and relieved the viral diseases caused by herpes simplex virus, influenza virus, and human immunodeficiency virus (HIV). Some mushroom compounds that act against HIV, influenza A virus, and hepatitis C virus showed antiviral effects comparable to those of antiviral drugs. Therefore, bioactive compounds from mushrooms could be candidates for treating viral infections. Citation: Seo, D.J.; Choi, C. Antiviral Bioactive Compounds of Mushrooms Keywords: mushroom; bioactive compound; virus; infection; antiviral mechanism and Their Antiviral Mechanisms: A Review. -

Intracellular Trafficking of HBV Particles
cells Review Intracellular Trafficking of HBV Particles Bingfu Jiang 1 and Eberhard Hildt 1,2,* 1 Department of Virology, Paul-Ehrlich-Institut, D-63225 Langen, Germany; [email protected] 2 German Center for Infection Research (DZIF), TTU Hepatitis, Marburg-Gießen-Langen, D63225 Langen, Germany * Correspondence: [email protected]; Tel.: +49-61-0377-2140 Received: 12 August 2020; Accepted: 2 September 2020; Published: 2 September 2020 Abstract: The human hepatitis B virus (HBV), that is causative for more than 240 million cases of chronic liver inflammation (hepatitis), is an enveloped virus with a partially double-stranded DNA genome. After virion uptake by receptor-mediated endocytosis, the viral nucleocapsid is transported towards the nuclear pore complex. In the nuclear basket, the nucleocapsid disassembles. The viral genome that is covalently linked to the viral polymerase, which harbors a bipartite NLS, is imported into the nucleus. Here, the partially double-stranded DNA genome is converted in a minichromosome-like structure, the covalently closed circular DNA (cccDNA). The DNA virus HBV replicates via a pregenomic RNA (pgRNA)-intermediate that is reverse transcribed into DNA. HBV-infected cells release apart from the infectious viral parrticle two forms of non-infectious subviral particles (spheres and filaments), which are assembled by the surface proteins but lack any capsid and nucleic acid. In addition, naked capsids are released by HBV replicating cells. Infectious viral particles and filaments are released via multivesicular bodies; spheres are secreted by the classic constitutive secretory pathway. The release of naked capsids is still not fully understood, autophagosomal processes are discussed. This review describes intracellular trafficking pathways involved in virus entry, morphogenesis and release of (sub)viral particles. -

Discovery of a Highly Divergent Hepadnavirus in Shrews from China
Virology 531 (2019) 162–170 Contents lists available at ScienceDirect Virology journal homepage: www.elsevier.com/locate/virology Discovery of a highly divergent hepadnavirus in shrews from China T Fang-Yuan Niea,b,1, Jun-Hua Tianc,1, Xian-Dan Lind,1, Bin Yuc, Jian-Guang Xinge, Jian-Hai Caof, ⁎ ⁎⁎ Edward C. Holmesg,h, Runlin Z. Maa, , Yong-Zhen Zhangb,h, a State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China b State Key Laboratory for Infectious Disease Prevention and Control; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases; Department of Zoonoses, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Changping, Beijing, China c Wuhan Center for Disease Control and Prevention, Wuhan, China d Wenzhou Center for Disease Control and Prevention, Wenzhou, Zhejiang Province, China e Wencheng Center for Disease Control and Prevention, Wencheng, Zhejiang Province, China f Longwan Center for Disease Control and Prevention, Longwan District, Wenzhou, Zhejiang Province, China g Marie Bashir Institute for Infectious Diseases and Biosecurity, Charles Perkins Centre, School of Life and Environmental Sciences and Sydney Medical School, The University of Sydney, Sydney, NSW 2006, Australia h Shanghai Public Health Clinical Center & Institute of Biomedical Sciences, Fudan University, Shanghai, China ARTICLE INFO ABSTRACT Keywords: Limited sampling means that relatively little is known about the diversity and evolutionary history of mam- Hepadnaviruses malian members of the Hepadnaviridae (genus Orthohepadnavirus). An important case in point are shrews, the Shrews fourth largest group of mammals, but for which there is limited knowledge on the role they play in viral evo- Phylogeny lution and emergence. -

Norovirus Infectious Agent Information Sheet
Norovirus Infectious Agent Information Sheet Introduction Noroviruses are non-enveloped (naked) RNA viruses with icosahedral nucleocapsid symmetry. The norovirus genome consists of (+) ssRNA, containing three open reading frames that encode for proteins required for transcription, replication, and assembly. There are five norovirus genogroups (GI-GV), and only GI, GII, and GIV infect humans. Norovirus belongs to the Caliciviridae family of viruses, and has had past names including, Norwalk virus and “winter-vomiting” disease. Epidemiology and Clinical Significance Noroviruses are considered the most common cause of outbreaks of non-bacterial gastroenteritis worldwide, are the leading cause of foodborne illness in the United States (58%), and account for 26% of hospitalizations and 10% of deaths associated with food consumption. Salad ingredients, fruit, and oysters are the most implicated in norovirus outbreaks. Aside from food and water, Noroviruses can also be transmitted by person to person contact and contact with environmental surfaces. The rapid spread of secondary infections occurs in areas where a large population is enclosed within a static environment, such as cruise ships, military bases, and institutions. Symptoms typically last for 24 to 48 hours, but can persist up to 96 hours in the immunocompromised. Pathogenesis, Immunity, Treatment and Prevention Norovirus is highly infectious due to low infecting dose, high excretion level (105 to 107 copies/mg stool), and continual shedding after clinical recovery (>1 month). The norovirus genome undergoes frequent change due to mutation and recombination, which increases its prevalence. Studies suggest that acquired immunity only last 6 months after infection. Gastroenteritis, an inflammation of the stomach and small and large intestines, is caused by norovirus infection. -

Viruses in Transplantation - Not Always Enemies
Viruses in transplantation - not always enemies Virome and transplantation ECCMID 2018 - Madrid Prof. Laurent Kaiser Head Division of Infectious Diseases Laboratory of Virology Geneva Center for Emerging Viral Diseases University Hospital of Geneva ESCMID eLibrary © by author Conflict of interest None ESCMID eLibrary © by author The human virome: definition? Repertoire of viruses found on the surface of/inside any body fluid/tissue • Eukaryotic DNA and RNA viruses • Prokaryotic DNA and RNA viruses (phages) 25 • The “main” viral community (up to 10 bacteriophages in humans) Haynes M. 2011, Metagenomic of the human body • Endogenous viral elements integrated into host chromosomes (8% of the human genome) • NGS is shaping the definition Rascovan N et al. Annu Rev Microbiol 2016;70:125-41 Popgeorgiev N et al. Intervirology 2013;56:395-412 Norman JM et al. Cell 2015;160:447-60 ESCMID eLibraryFoxman EF et al. Nat Rev Microbiol 2011;9:254-64 © by author Viruses routinely known to cause diseases (non exhaustive) Upper resp./oropharyngeal HSV 1 Influenza CNS Mumps virus Rhinovirus JC virus RSV Eye Herpes viruses Parainfluenza HSV Measles Coronavirus Adenovirus LCM virus Cytomegalovirus Flaviviruses Rabies HHV6 Poliovirus Heart Lower respiratory HTLV-1 Coxsackie B virus Rhinoviruses Parainfluenza virus HIV Coronaviruses Respiratory syncytial virus Parainfluenza virus Adenovirus Respiratory syncytial virus Coronaviruses Gastro-intestinal Influenza virus type A and B Human Bocavirus 1 Adenovirus Hepatitis virus type A, B, C, D, E Those that cause -

Risk Groups: Viruses (C) 1988, American Biological Safety Association
Rev.: 1.0 Risk Groups: Viruses (c) 1988, American Biological Safety Association BL RG RG RG RG RG LCDC-96 Belgium-97 ID Name Viral group Comments BMBL-93 CDC NIH rDNA-97 EU-96 Australia-95 HP AP (Canada) Annex VIII Flaviviridae/ Flavivirus (Grp 2 Absettarov, TBE 4 4 4 implied 3 3 4 + B Arbovirus) Acute haemorrhagic taxonomy 2, Enterovirus 3 conjunctivitis virus Picornaviridae 2 + different 70 (AHC) Adenovirus 4 Adenoviridae 2 2 (incl animal) 2 2 + (human,all types) 5 Aino X-Arboviruses 6 Akabane X-Arboviruses 7 Alastrim Poxviridae Restricted 4 4, Foot-and- 8 Aphthovirus Picornaviridae 2 mouth disease + viruses 9 Araguari X-Arboviruses (feces of children 10 Astroviridae Astroviridae 2 2 + + and lambs) Avian leukosis virus 11 Viral vector/Animal retrovirus 1 3 (wild strain) + (ALV) 3, (Rous 12 Avian sarcoma virus Viral vector/Animal retrovirus 1 sarcoma virus, + RSV wild strain) 13 Baculovirus Viral vector/Animal virus 1 + Togaviridae/ Alphavirus (Grp 14 Barmah Forest 2 A Arbovirus) 15 Batama X-Arboviruses 16 Batken X-Arboviruses Togaviridae/ Alphavirus (Grp 17 Bebaru virus 2 2 2 2 + A Arbovirus) 18 Bhanja X-Arboviruses 19 Bimbo X-Arboviruses Blood-borne hepatitis 20 viruses not yet Unclassified viruses 2 implied 2 implied 3 (**)D 3 + identified 21 Bluetongue X-Arboviruses 22 Bobaya X-Arboviruses 23 Bobia X-Arboviruses Bovine 24 immunodeficiency Viral vector/Animal retrovirus 3 (wild strain) + virus (BIV) 3, Bovine Bovine leukemia 25 Viral vector/Animal retrovirus 1 lymphosarcoma + virus (BLV) virus wild strain Bovine papilloma Papovavirus/ -

Scwds Briefs
SCWDS BRIEFS A Quarterly Newsletter from the Southeastern Cooperative Wildlife Disease Study College of Veterinary Medicine The University of Georgia Phone (706) 542 - 1741 Athens, Georgia 30602 FAX (706) 542-5865 Volume 36 April 2020 Number 1 Working Together: The 40th Anniversary of limited HD reports probably relate to enzootic the National Hemorrhagic Disease Survey stability; on the northern edge, the low frequency of reports is likely driven by limited EHDV and BTV In the last issue of the SCWDS BRIEFS, hemorrhagic transmission due to either the absence of vectors or disease (HD) report data from Indiana, Ohio, environmental conditions that reduce vector numbers Kentucky, and West Virginia were highlighted to or their ability to transmit these viruses. Within these demonstrate how these long-term data can help states, east-west gradients in HD reporting also are detect and map the northern expansion of HD over evident. The factors that drive this within-state time. In addition to providing a means to detect variation are not well understood and possibly relate temporal changes in HD patterns, this long-term data to habitat gradients and complex set provides a window to better understand spatial vector/host/environmental interactions that are patterns and risks within areas where HD has unique to each individual state. historically occurred. In part two of this four-part series, we will explore HD patterns in another area – the Great Plains, where HD is commonly reported and large-scale outbreaks occasionally occur. These states were included in the survey in 1982, and thanks to their continued support, this data set now spans 38 years. -

Norovirus in Healthcare Facilities Fact Sheet Released December 21, 2006
Norovirus in Healthcare Facilities Fact Sheet Released December 21, 2006 Diagnosis of norovirus infection Diagnosis of norovirus infection relies on the detection of viral RNA in the stools of affected persons, by use of reverse transcription- polymerase chain reaction (RT-PCR) assays. This technology is available at CDC and most state public health laboratories and should be considered in the event of outbreaks of gastroenteritis in healthcare facilities. Identification of the virus can be best made from stool specimens taken within 48 to 72 hours after onset of symptoms, although good results can be obtained by using RTPCR on General Information samples taken as long as 7 days after symptom onset. Other methods of diagnosis, usually only available in research settings, include Virology electron microscopy and serologic assays for a rise in titer in paired Noroviruses (genus Norovirus, family Caliciviridae) are a sera collected at least three weeks apart. Commercial enzyme-linked group of related, single-stranded RNA, non-enveloped immunoassays are available but are of relatively low sensitivity, so viruses that cause acute gastroenteritis in humans. their use is limited to diagnosis of the etiology of outbreaks. Because Norovirus was recently approved as the official genus name of the limited availability of timely and routine laboratory diagnostic for the group of viruses provisionally described as “Norwalk- methods, a clinical diagnosis of norovirus infection is often used, like viruses” (NLV). Currently, human noroviruses belong to especially when other agents of gastroentertis have been ruled out. one of three norovirus genogroups (GI, GII, or GIV), each of which is further divided into >25 genetic clusters. -

Arenaviridae Astroviridae Filoviridae Flaviviridae Hantaviridae
Hantaviridae 0.7 Filoviridae 0.6 Picornaviridae 0.3 Wenling red spikefish hantavirus Rhinovirus C Ahab virus * Possum enterovirus * Aronnax virus * * Wenling minipizza batfish hantavirus Wenling filefish filovirus Norway rat hunnivirus * Wenling yellow goosefish hantavirus Starbuck virus * * Porcine teschovirus European mole nova virus Human Marburg marburgvirus Mosavirus Asturias virus * * * Tortoise picornavirus Egyptian fruit bat Marburg marburgvirus Banded bullfrog picornavirus * Spanish mole uluguru virus Human Sudan ebolavirus * Black spectacled toad picornavirus * Kilimanjaro virus * * * Crab-eating macaque reston ebolavirus Equine rhinitis A virus Imjin virus * Foot and mouth disease virus Dode virus * Angolan free-tailed bat bombali ebolavirus * * Human cosavirus E Seoul orthohantavirus Little free-tailed bat bombali ebolavirus * African bat icavirus A Tigray hantavirus Human Zaire ebolavirus * Saffold virus * Human choclo virus *Little collared fruit bat ebolavirus Peleg virus * Eastern red scorpionfish picornavirus * Reed vole hantavirus Human bundibugyo ebolavirus * * Isla vista hantavirus * Seal picornavirus Human Tai forest ebolavirus Chicken orivirus Paramyxoviridae 0.4 * Duck picornavirus Hepadnaviridae 0.4 Bildad virus Ned virus Tiger rockfish hepatitis B virus Western African lungfish picornavirus * Pacific spadenose shark paramyxovirus * European eel hepatitis B virus Bluegill picornavirus Nemo virus * Carp picornavirus * African cichlid hepatitis B virus Triplecross lizardfish paramyxovirus * * Fathead minnow picornavirus -
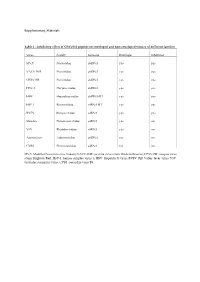
Inhibitory Effect of CPXV012 Peptide on Enveloped and Non-Enveloped Viruses of Different Families
Supplementary Materials Table 1 - Inhibitory effect of CPXV012 peptide on enveloped and non-enveloped viruses of different families Virus Family Genome Envelope Inhibition MVA Poxviridae dsDNA yes yes VACV-WR Poxviridae dsDNA yes yes CPXV-BR Poxviridae dsDNA yes yes HSV-1 Herpesviridae dsDNA yes yes HBV Hepadnaviridae dsDNA-RT yes yes HIV-1 Retroviridae ssRNA-RT yes yes RVFV Bunyaviridae ssRNA yes yes Measles Paramyxoviridae ssRNA yes no VSV Rhabdoviridae ssRNA yes no Adenovirus Adenoviridae dsDNA no no CVB3 Picornaviridae ssRNA no no MVA: Modified Vaccinia virus Ankara; VACV-WR: vaccinia virus strain Western Reserve; CPXV-BR: cowpox virus strain Brighton Red; HSV-1: herpes simplex virus-1; HBV: Hepatitis B virus; RVFV: Rift Valley fever virus; VSV: vesicular stomatitis virus; CVB3: coxsackie virus B3. A B 140 0.6 ) l 120 ro t 100 on 0.4 c g f 80 µ / o 60 % NR ( 0.2 1 - 40 T S 20 W 0 0.0 O 5 0 0 0 5 0 0 0 S 2 5 0 0 O 2 5 0 0 1 2 S 1 2 M M D D CPXV012 peptide [ g/mL] CPXV012 peptide [ g/mL] C ) . 30000 U . A ( e u 20000 l b r e t i t 10000 ll e C 0 0 50 100 150 200 CPXV012 peptide [ g/mL] Huh7.5 Vero Figure S1: CPXV012 peptide does not affect cell viability. (A-C) Cells were treated with the indicated concentrations of peptide or DMSO as vehicle control. S.E.M. of three independent experiments is shown. (A) Viability of MJS cells was measured by WST-1 assay or (B) Neutral Red uptake. -

Mechanism of Hepatitis B Virus Cccdna Formation
viruses Review Mechanism of Hepatitis B Virus cccDNA Formation Lei Wei and Alexander Ploss * 110 Lewis Thomas Laboratory, Department of Molecular Biology, Princeton University, Washington Road, Princeton, NJ 08544, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-609-258-7128 Abstract: Hepatitis B virus (HBV) remains a major medical problem affecting at least 257 million chronically infected patients who are at risk of developing serious, frequently fatal liver diseases. HBV is a small, partially double-stranded DNA virus that goes through an intricate replication cycle in its native cellular environment: human hepatocytes. A critical step in the viral life-cycle is the conversion of relaxed circular DNA (rcDNA) into covalently closed circular DNA (cccDNA), the latter being the major template for HBV gene transcription. For this conversion, HBV relies on multiple host factors, as enzymes capable of catalyzing the relevant reactions are not encoded in the viral genome. Combinations of genetic and biochemical approaches have produced findings that provide a more holistic picture of the complex mechanism of HBV cccDNA formation. Here, we review some of these studies that have helped to provide a comprehensive picture of rcDNA to cccDNA conversion. Mechanistic insights into this critical step for HBV persistence hold the key for devising new therapies that will lead not only to viral suppression but to a cure. Keywords: hepatitis B virus; HBV; viral replication; cccDNA biogenesis; rcDNA; DNA repair Citation: Wei, L.; Ploss, A. 1. Overview of HBV Life Cycle and cccDNA Biogenesis Mechanism of Hepatitis B Virus cccDNA Formation. Viruses 2021, 13, The hepatotropic HBV belongs to the Hepadnaviridae family and is a blood-borne 1463. -

Downloaded from Transcriptome Shotgun Assembly (TSA) Database on 29 November 2020 (Ftp://Ftp.Ddbj.Nig.Ac.Jp/Ddbj Database/Tsa/, Table S3)
viruses Article Discovery and Characterization of Actively Replicating DNA and Retro-Transcribing Viruses in Lower Vertebrate Hosts Based on RNA Sequencing Xin-Xin Chen, Wei-Chen Wu and Mang Shi * School of Medicine, Sun Yat-sen University, Shenzhen 518107, China; [email protected] (X.-X.C.); [email protected] (W.-C.W.) * Correspondence: [email protected] Abstract: In a previous study, a metatranscriptomics survey of RNA viruses in several important lower vertebrate host groups revealed huge viral diversity, transforming the understanding of the evolution of vertebrate-associated RNA virus groups. However, the diversity of the DNA and retro-transcribing viruses in these host groups was left uncharacterized. Given that RNA sequencing is capable of revealing viruses undergoing active transcription and replication, we collected previously generated datasets associated with lower vertebrate hosts, and searched them for DNA and retro-transcribing viruses. Our results revealed the complete genome, or “core gene sets”, of 18 vertebrate-associated DNA and retro-transcribing viruses in cartilaginous fishes, ray- finned fishes, and amphibians, many of which had high abundance levels, and some of which showed systemic infections in multiple organs, suggesting active transcription or acute infection within the host. Furthermore, these new findings recharacterized the evolutionary history in the families Hepadnaviridae, Papillomaviridae, and Alloherpesviridae, confirming long-term virus–host codivergence relationships for these virus groups.