Comparison of Efficacy and Safety Between Benidipine And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of New Drugs Approved in India from 1991 to 2000
LIST OF NEW DRUGS APPROVED IN INDIA FROM 1991 TO 2000 S. No Name of Drug Pharmacological action/ Date of Indication Approval 1 Ciprofloxacin 0.3% w/v Eye Indicated in the treatment of February-1991 Drops/Eye Ointment/Ear Drop external ocular infection of the eye. 2 Diclofenac Sodium 1gm Gel March-1991 3 i)Cefaclor Monohydrate Antibiotic- In respiratory April-1991 250mg/500mg Capsule. infections, ENT infection, UT ii)Cefaclor Monohydrate infections, Skin and skin 125mg/5ml & 250mg/5ml structure infections. Suspension. iii)Cefaclor Monohydrate 100mg/ml Drops. iv)Cefaclor 187mg/5ml Suspension (For paediatric use). 4 Sheep Pox Vaccine (For April-1991 Veterinary) 5 Omeprazole 10mg/20mg Short term treatment of April-1991 Enteric Coated Granules duodenal ulcer, gastric ulcer, Capsule reflux oesophagitis, management of Zollinger- Ellison syndrome. 6 i)Nefopam Hydrochloride Non narcotic analgesic- Acute April-1991 30mg Tablet. and chronic pain, including ii)Nefopam Hydrochloride post-operative pain, dental 20mg/ml Injection. pain, musculo-skeletal pain, acute traumatic pain and cancer pain. 7 Buparvaquone 5% w/v Indicated in the treatment of April-1991 Solution for Injection (For bovine theileriosis. Veterinary) 8 i)Kitotifen Fumerate 1mg Anti asthmatic drug- Indicated May-1991 Tablet in prophylactic treatment of ii)Kitotifen Fumerate Syrup bronchial asthma, symptomatic iii)Ketotifen Fumerate Nasal improvement of allergic Drops conditions including rhinitis and conjunctivitis. 9 i)Pefloxacin Mesylate Antibacterial- In the treatment May-1991 Dihydrate 400mg Film Coated of severe infection in adults Tablet caused by sensitive ii)Pefloxacin Mesylate microorganism (gram -ve Dihydrate 400mg/5ml Injection pathogens and staphylococci). iii)Pefloxacin Mesylate Dihydrate 400mg I.V Bottles of 100ml/200ml 10 Ofloxacin 100mg/50ml & Indicated in RTI, UTI, May-1991 200mg/100ml vial Infusion gynaecological infection, skin/soft lesion infection. -

Dorset Medicines Advisory Group
DORSET CARDIOLOGY WORKING GROUP GUIDELINE FOR CALCIUM CHANNEL BLOCKERS IN HYPERTENSION SUMMARY The pan-Dorset cardiology working group continues to recommend the use of amlodipine (a third generation dihydropyridine calcium-channel blocker) as first choice calcium channel blocker on the pan-Dorset formulary for hypertension. Lercanidipine is second choice, lacidipine third choice and felodipine is fourth choice. This is due to preferable side effect profiles in terms of ankle oedema and relative costs of the preparations. Note: where angina is the primary indication or is a co-morbidity prescribers must check against the specific product characteristics (SPC) for an individual drug to confirm this is a licensed indication. N.B. Lacidipine and lercandipine are only licensed for use in hypertension. Chapter 02.06.02 CCBs section of the Formulary has undergone an evidence-based review. A comprehensive literature search was carried out on NHS Evidence, Medline, EMBASE, Cochrane Database, and UK Duets. This was for recent reviews or meta-analyses on calcium channel blockers from 2009 onwards (comparative efficacy and side effects) and randomised controlled trials (RCTs). REVIEW BACKGROUND Very little good quality evidence exists. No reviews, meta-analyses or RCTs were found covering all calcium channel blockers currently on the formulary. Another limitation was difficulty obtaining full text original papers for some of the references therefore having to use those from more obscure journals instead. Some discrepancies exist between classification of generations of dihydropyridine CCBs, depending upon the year of publication of the reference/authors’ interpretation. Dihydropyridine (DHP) CCBs tend to be more potent vasodilators than non-dihydropyridine (non-DHP) CCBs (diltiazem, verapamil), but the latter have greater inotropic effects. -

Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Cr
Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Crizotinib (PF-02341066) 1 4 55 Docetaxel 1 5 98 Anastrozole 1 6 25 Cladribine 1 7 23 Methotrexate 1 8 -187 Letrozole 1 9 65 Entecavir Hydrate 1 10 48 Roxadustat (FG-4592) 1 11 19 Imatinib Mesylate (STI571) 1 12 0 Sunitinib Malate 1 13 34 Vismodegib (GDC-0449) 1 14 64 Paclitaxel 1 15 89 Aprepitant 1 16 94 Decitabine 1 17 -79 Bendamustine HCl 1 18 19 Temozolomide 1 19 -111 Nepafenac 1 20 24 Nintedanib (BIBF 1120) 1 21 -43 Lapatinib (GW-572016) Ditosylate 1 22 88 Temsirolimus (CCI-779, NSC 683864) 1 23 96 Belinostat (PXD101) 1 24 46 Capecitabine 1 25 19 Bicalutamide 1 26 83 Dutasteride 1 27 68 Epirubicin HCl 1 28 -59 Tamoxifen 1 29 30 Rufinamide 1 30 96 Afatinib (BIBW2992) 1 31 -54 Lenalidomide (CC-5013) 1 32 19 Vorinostat (SAHA, MK0683) 1 33 38 Rucaparib (AG-014699,PF-01367338) phosphate1 34 14 Lenvatinib (E7080) 1 35 80 Fulvestrant 1 36 76 Melatonin 1 37 15 Etoposide 1 38 -69 Vincristine sulfate 1 39 61 Posaconazole 1 40 97 Bortezomib (PS-341) 1 41 71 Panobinostat (LBH589) 1 42 41 Entinostat (MS-275) 1 43 26 Cabozantinib (XL184, BMS-907351) 1 44 79 Valproic acid sodium salt (Sodium valproate) 1 45 7 Raltitrexed 1 46 39 Bisoprolol fumarate 1 47 -23 Raloxifene HCl 1 48 97 Agomelatine 1 49 35 Prasugrel 1 50 -24 Bosutinib (SKI-606) 1 51 85 Nilotinib (AMN-107) 1 52 99 Enzastaurin (LY317615) 1 53 -12 Everolimus (RAD001) 1 54 94 Regorafenib (BAY 73-4506) 1 55 24 Thalidomide 1 56 40 Tivozanib (AV-951) 1 57 86 Fludarabine -

Medicine for Prevention of and Treatment for Arteriosclerosis and Hypertension
Europäisches Patentamt *EP001604664A1* (19) European Patent Office Office européen des brevets (11) EP 1 604 664 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC (43) Date of publication: (51) Int Cl.7: A61K 31/4422, A61K 45/06, 14.12.2005 Bulletin 2005/50 A61P 9/00, A61P 9/10, A61P 43/00, A61P 9/12, (21) Application number: 04706359.9 A61P 13/12 (22) Date of filing: 29.01.2004 (86) International application number: PCT/JP2004/000861 (87) International publication number: WO 2004/067003 (12.08.2004 Gazette 2004/33) (84) Designated Contracting States: • IWAI, Masaru AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Shigenobu-cho, Onsen-gun, HU IE IT LI LU MC NL PT RO SE SI SK TR Ehime 791-0204 (JP) Designated Extension States: • SADA, Toshio, Sankyo Company, Limited AL LT LV MK Tokyo 140-8710 (JP) • MIZUNO, Makoto, Sankyo Company, Limited (30) Priority: 31.01.2003 JP 2003022990 Tokyo 140-8710 (JP) 07.02.2003 JP 2003030830 (74) Representative: Gibson, Christian John Robert (71) Applicant: Sankyo Company, Limited Marks & Clerk Tokyo 103-8426 (JP) 90 Long Acre London WC2E 9RA (GB) (72) Inventors: • HORIUCHI, Masatsugu Onsen-gun, Ehime 791-0204 (JP) (54) MEDICINE FOR PREVENTION OF AND TREATMENT FOR ARTERIOSCLEROSIS AND HYPERTENSION (57) A medicament comprising the following composition: (A) an angiotensin II receptor antagonist selected from the group of a compound having a general formula (I), pharmacologically acceptable esters thereof and pharmacolog- ically acceptable salts thereof (for example, olmesartan medoxomil and the like); EP 1 604 664 A1 Printed by Jouve, 75001 PARIS (FR) (Cont. -

Antioxidant Effects of Calcium Antagonists Rat Myocardial
223 Antioxidant Effects of Calcium Antagonists ['Ii] Rat Myocardial Membrane Lipid Peroxidation Hitoshi Sugawara, Katsuyuki Tobise, and Kenjiro Kikuchi We studied the antioxidant effects of nine calcium antagonists (nisoldipine, benidipine, nilvadipine, felo- dipine, nicardipine; nitrendipine, nifedipine, verapamil, and diltiazem) by means of rat myocardial membrane lipid peroxidation with a nonenzymatic active oxygen-generating system (DHF/FeC13-ADP). The order of antioxidant potency of these agents was nilvadipine > nisoldipine > felodipine > nicardipine > verapamil > benidipine. Their IC50 values (,uM) were 25.1, 28.2, 42.0, 150.0, 266.1, and 420.0, re- spectively. In contrast, nitrendipine, nifedipine, and diltiazem had little inhibitory effect on lipid peroxi- dation.These six calcium antagonists could be divided into four types on the basis of their antioxidant mechanisms. Nilvadipine, nisoldipine, and verapamil, which showed antioxidant effects both before and after the addition of active oxygen, and reduced the dihydroxyfumarate (DHF) auto-oxidation rate, were chain-breaking and preventive antioxidants. Felodipine, which showed antioxidant effects both before and after exposure to active oxygen and increased the DHF auto-oxidation rate, was only a chain-break- ing antioxidant. Nicardipine, which showed an antioxidant effect only before exposure to active oxygen and reduced the DHF auto-oxidation rate, was mainly a preventive antioxidant. Benidipine, which showed an antioxidant effect only before exposure to active oxygen and had no appreciable effect on the DHF auto-oxidation rate, could interrupt the chain reaction of lipid peroxidation at the initial step alone. Although these results suggest that the antioxidant properties of some calcium antagonists may be beneficial clinically in protecting against cellular damage caused by lipid peroxidation, further studies are required to establish the antioxidant effects of these agents in vivo. -

Benidipine, a Dihydropyridine-Ca2+ Channel Blocker, Increases the Endothelial Differentiation of Endothelial Progenitor Cells in Vitro
1047 Hypertens Res Vol.29 (2006) No.12 p.1047-1054 Original Article Benidipine, a Dihydropyridine-Ca2+ Channel Blocker, Increases the Endothelial Differentiation of Endothelial Progenitor Cells In Vitro Hiroshi ANDO1), Kosuke NAKANISHI2), Mami SHIBATA1), Kazuhide HASEGAWA2), Kozo YAO2), and Hiromasa MIYAJI1) Benidipine is a dihydropyridine-Ca2+ channel blocker used in the treatment of hypertension and angina pec- toris. In the present study, we examined the effects of benidipine on the endothelial differentiation of circu- lating endothelial progenitor cells (EPCs) using an in vitro culture method. Peripheral blood derived mononuclear cells (PBMCs) containing EPCs were isolated from C57BL/6 mice, and then the cells were cul- tured on vitronectin/gelatin-coated slide glasses. After 7 days of culture, endothelial cells differentiated from EPCs were identified as adherent cells with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine–labeled acetylated low density lipoprotein (DiI-Ac-LDL) uptake and lectin binding under a fluorescent microscope. Incubation of PBMCs for 7 days with benidipine (0.01–1 µmol/l) significantly increased the number of DiI- Ac-LDL+/fluorescein isothiocyanate-lectin (FITC-Lectin)+ cells. Wortmannin, a phosphoinositide-3 kinase (PI3K) inhibitor, selectively attenuated the effect of benidipine on the endothelial differentiation. In addition, benidipine treatment augmented the phosphorylation of Akt, indicating that the PI3K/Akt pathway contrib- uted, at least in part, to the endothelial differentiation induced by benidipine. These results suggest that the treatment with benidipine may increase the endothelial differentiation of circulating EPCs and contribute to endothelial protection, prevention of cardiovascular disease, and/or an improvement of the prognosis after ischemic damage. -

Benidipine Reduces Albuminuria and Plasma Aldosterone in Mild-To-Moderate Stage Chronic Kidney Disease with Albuminuria
Hypertension Research (2011) 34, 268–273 & 2011 The Japanese Society of Hypertension All rights reserved 0916-9636/11 $32.00 www.nature.com/hr ORIGINAL ARTICLE Benidipine reduces albuminuria and plasma aldosterone in mild-to-moderate stage chronic kidney disease with albuminuria Masanori Abe1, Kazuyoshi Okada1, Noriaki Maruyama1, Shiro Matsumoto1, Takashi Maruyama1, Takayuki Fujita1, Koichi Matsumoto1 and Masayoshi Soma1,2 Benidipine inhibits both L- and T-type Ca channels, and has been shown to dilate the efferent arterioles as effectively as the afferent arterioles. In this study, we conducted an open-label and randomized trial to compare the effects of benidipine with those of amlodipine on blood pressure (BP), albuminuria and aldosterone concentration in hypertensive patients with mild-to- moderate stage chronic kidney disease (CKD). Patients with BPX130/80 mm Hg, with estimated glomerular filtration rate (eGFR) of 30–90 ml minÀ1 per 1.73 m2, and with albuminuria430 mg per g creatinine (Cr), despite treatment with the maximum recommended dose of angiotensin II receptor blockers (ARBs) were randomly assigned to two groups. Patients received either of the following two treatment regimens: 2 mg per day benidipine, which was increased up to a dose of 8 mg per day (n¼52), or 2.5 mg per day amlodipine, which was increased up to a dose of 10 mg per day (n¼52). After 6 months of treatment, a significant and comparable reduction in the systolic and diastolic BP was observed in both groups. The decrease in the urinary albumin to Cr ratio in the benidipine group was significantly lower than that in the amlodipine group. -

Beneficial Effects of Low-Dose Benidipine in Acute Autoimmune
EXPERIMENTAL INVESTIGATION Circ J 2003; 67: 545–550 Beneficial Effects of Low-Dose Benidipine in Acute Autoimmune Myocarditis Suppressive Effects on Inflammatory Cytokines and Inducible Nitric Oxide Synthase Zuyi Yuan, MD; Chiharu Kishimoto, MD; Keisuke Shioji, MD Excessive production of nitric oxide (NO) by inducible NO synthase (iNOS) contributes to the progression of myocardial damage in myocarditis. Some dihydropyridine calcium channel blockers reportedly inhibit NO production and proinflammatory cytokines and the present study sought to clarify if a low dose of benidipine, a novel dihydropyridine calcium channel blocker, would ameliorate experimental autoimmune myocarditis (EAM). Rats with or without myocarditis were administered oral benidipine at a dose of 3mg·kg–1·day–1 for 3 weeks. Low-dose benidipine did not decrease blood pressure significantly compared with the untreated group, but markedly reduced the severity of myocarditis. Myocardial interleukin-1β(IL-1β) expression and IL-1β-posi- tive cells were significantly less in rats with EAM that were treated with low-dose benidipine compared with un- treated rats. Also, myocardial iNOS expression and iNOS-positive cells were markedly reduced in in the treated rats compared with the untreated group. Furthermore, myocardial NO production and nitrotyrosine expression were suppressed by the treatment in rats with EAM. The cardioprotection of low-dose benidipine may be caused by suppression of inflammatory cytokines and inhibition of NO production. (Circ J 2003; 67: 545–550) Key Words: Calcium -

Pharmacokinetic Drug–Drug Interactions
Therapeutics and Clinical Risk Management Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Pharmacokinetic drug–drug interactions between 1,4-dihydropyridine calcium channel blockers and statins: factors determining interaction strength and relevant clinical risk management Yi-Ting Zhou1 Background: Coadministration of 1,4-dihydropyridine calcium channel blockers (DHP-CCBs) Lu-Shan Yu2 with statins (or 3-hydroxy-3-methylglutaryl-coenzyme A [HMG-CoA] reductase inhibitors) Su Zeng2 is common for patients with hypercholesterolemia and hypertension. To reduce the risk of Yu-Wen Huang1 myopathy, in 2011, the US Food and Drug Administration (FDA) Drug Safety Communication Hui-Min Xu1 set a new dose limitation for simvastatin, for patients taking simvastatin concomitantly with Quan Zhou1 amlodipine. However, there is no such dose limitation for atorvastatin for patients receiving amlodipine. The combination pill formulation of amlodipine/atorvastatin is available on the 1 Department of Pharmacy, the Second market. There been no systematic review of the pharmacokinetic drug–drug interaction (DDI) Affiliated Hospital, School of Medicine, 2Department of Pharmaceutical profile of DHP-CCBs with statins, the underlying mechanisms for DDIs of different degree, or Analysis and Drug Metabolism, the corresponding management of clinical risk. College of Pharmaceutical Sciences, The relevant literature was identified by performing a PubMed search, covering Zhejiang University, Hangzhou, Methods: Zhejiang Province, People’s Republic the period from January 1987 to September 2013. Studies in the field of drug metabolism and of China pharmacokinetics that described DDIs between DHP-CCB and statin or that directly com- pared the degree of DDIs associated with cytochrome P450 (CYP)3A4-metabolized statins or DHP-CCBs were included. -

187 Scientists Repurposing FDA-Approved Drugs to Fight
Vol.33 No.3 2019 Science Watch Life Sciences Scientists Repurposing FDA- Approved Drugs to Fight against Viruses n a study published in Cell Research on August 23, researchers from the CAS Wuhan Institute Iof Virology/Center for Biosafety Mega-Science, Chinese Academy of Sciences, together with their collaborator from State Key Laboratory of Pathogen and Biosecurity discovered a new talent for two molecules of calcium channel blockers (CCBs), a group of widely used anti-hypertensive and anti-atherosclerotic agents. These two CCB molecules were found to inhibit the replication of a certain virus that causes severe fever with thrombocytopenia syndrome (SFTS). SFTS is an emerging tick-borne infectious disease caused by a novel phlebovirus (SFTS virus, SFTSV), which was listed among the top 10 priority infectious A proposed model for the anti-SFTSV activity of the two calcium diseases by the World Health Organization due to its high channel blockers that inhibit viral internalization and replication through reducing the intracellular Ca2+ concentration. (Credit: Prof. fatality and pandemic risk. Currently, there is no medicine PENG Ke’s group) marketed specifically against SFTSV. In the face of a growing threat to public health imposed by SFTSV, a fight back against SFTSV is highly demanded. hydrochloride inhibits virus infection through impairing To meet the demanding need, researchers resorted virus internalization and genome replication through to screen FDA-approved drugs for anti-viral compounds. reducing the intracellular Ca2+ level. This is an effective strategy to repurpose drug application They also tested a broad panel of CCBs to see whether as it eases one’s mind on the safety concerns of the inhibition of SFTSV replication is a general feature of identified drug candidates. -
PDF-Document
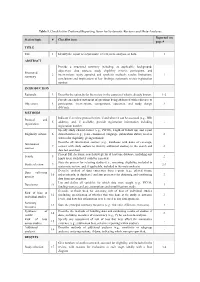
Table 1. Checklist for Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Reported on Section/topic # Checklist item page # TITLE Title 1 Identify the report as a systematic review, meta-analysis, or both. 1 ABSTRACT Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and Structured 2 interventions; study appraisal and synthesis methods; results; limitations; 1 summary conclusions and implications of key findings; systematic review registration number. INTRODUCTION Rationale 3 Describe the rationale for the review in the context of what is already known. 1-2 Provide an explicit statement of questions being addressed with reference to Objectives 4 participants, interventions, comparisons, outcomes, and study design 2 (PICOS). METHODS Indicate if a review protocol exists, if and where it can be accessed (e.g., Web Protocol and 5 address), and, if available, provide registration information including - registration registration number. Specify study characteristics (e.g., PICOS, length of follow-up) and report Eligibility criteria 6 characteristics (e.g., years considered, language, publication status) used as 2 criteria for eligibility, giving rationale. Describe all information sources (e.g., databases with dates of coverage, Information 7 contact with study authors to identify additional studies) in the search and 2 sources date last searched. Present full electronic search strategy for at least one database, including any Search 8 2 limits used, such that it could be repeated. State the process for selecting studies (i.e., screening, eligibility, included in Study selection 9 2-3 systematic review, and, if applicable, included in the meta-analysis). Describe method of data extraction from reports (e.g., piloted forms, Data collection 10 independently, in duplicate) and any processes for obtaining and confirming 3 process data from investigators. -

Incidence of Gingival Overgrowth Caused by Calcium Channel Blockers
Vol.27 No.2 2008 歯 薬 療 法79 Present research status on drug-induced gingival overgrowth Incidence of gingival overgrowth caused by calcium channel blockers MAKIKO ONO1, NAOKO OHNO1, KAZUHIRO HASEGAWA1, SHIGEO TANAKA1, MASAMICHI KOMIYA1, HIROKO MATSUMOTO2, AKIRA FUJII2 and YOSHIAKI AKIMOTO1 Abstract : The incidence of gingival overgrowth caused by calcium channel blockers was determined. The overgrowth was found in patients receiving amlodipine, diltiazem, manidipine, nicardipine, nifedipine and nisoldipine. The highest rate of gingival overgrowth was obtained by nifedipine (7.6%), followed by diltiazem (4.1%), manidipine (1.8%), amlodipine (1.l%), nisoldipine (1.1%) and nicardipine (0.5%). The rate of nifedipine-induced gingival overgrowth was significant- ly higher than those of amlodipine, manidipine, nicardipine and nisoldipine, but not diltiazem. Key words : calcium channel blocker, gingival overgrowth, incidence treatment of their various oral diseases. The patients Introduction were surveyed to determine the calcium channel Gingival overgrowth induced by calcium channel blocker-induced gingival overgrowth. The 15 kinds of blockers is a well-known adverse effect. Amlodipine1-3), calcium channel blocker and numbers of cases were as diltiazem4,5), felodipine6), manidipine7,8) nicardipine9), follows: amlodipine (n=267), azelnipine (n=11), barni- nifedipine3-5.8.10-12)nisoldipine13), nitrendipine14) and dipine (n=25), benidipine (n=28), diltiazem (n= verapamil3-5,15.16) were reported as causative drugs 196), efonidipine (n=14), felodipine (n=4), flunarizine for gingival overgrowth. However, this evidence has (n=32), manidipine (n=111), nicardipine (n=219), come from several case reports, and there have been nifedipine (n=347), nilvadipine (n=58), nisoldipine few prevalence studyies to evaluate the magnitude of (n=89), nitrendipine (n=25) or verapamil (n=41).