Healthcare Worker Vaccination Recommendations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety of Immunization During Pregnancy a Review of the Evidence
Safety of Immunization during Pregnancy A review of the evidence Global Advisory Committee on Vaccine Safety © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

Mmrv Vaccine
VACCINE INFORMATION STATEMENT (Measles, Mumps, Many Vaccine Information Statements are available in Spanish and other languages. MMRV Vaccine Rubella and See www.immunize.org/vis Varicella) Hojas de información sobre vacunas están disponibles en español y en muchos otros What You Need to Know idiomas. Visite www.immunize.org/vis These are recommended ages. But children can get the Measles, Mumps, Rubella and second dose up through 12 years as long as it is at least 1 Varicella 3 months after the first dose. Measles, Mumps, Rubella, and Varicella (chickenpox) can be serious diseases: Children may also get these vaccines as 2 separate shots: MMR (measles, mumps and rubella) and Measles varicella vaccines. • Causes rash, cough, runny nose, eye irritation, fever. • Can lead to ear infection, pneumonia, seizures, brain 1 Shot (MMRV) or 2 Shots (MMR & Varicella)? damage, and death. • Both options give the same protection. Mumps • One less shot with MMRV. • Causes fever, headache, swollen glands. • Children who got the first dose as MMRV have • Can lead to deafness, meningitis (infection of the brain had more fevers and fever-related seizures (about and spinal cord covering), infection of the pancreas, 1 in 1,250) than children who got the first dose as painful swelling of the testicles or ovaries, and, rarely, separate shots of MMR and varicella vaccines on death. the same day (about 1 in 2,500). Rubella (German Measles) Your doctor can give you more information, • Causes rash and mild fever; and can cause arthritis, including the Vaccine Information Statements for (mostly in women). MMR and Varicella vaccines. -

Proquad, INN-Measles, Mumps, Rubella and Varicella Vaccine (Live)
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ProQuad powder and solvent for suspension for injection ProQuad powder and solvent for suspension for injection in a pre-filled syringe Measles, mumps, rubella and varicella vaccine (live). 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, one dose (0.5 mL) contains: 1 Measles virus Enders’ Edmonston strain (live, attenuated) ........ not less than 3.00 log10 TCID * 50 1 Mumps virus Jeryl Lynn™ (Level B) strain (live, attenuated) ... not less than 4.30 log10 TCID * 50 2 Rubella virus Wistar RA 27/3 strain (live, attenuated)............... not less than 3.00 log10 TCID * 50 3 Varicella virus Oka/Merck strain (live, attenuated) ................... not less than 3.99 log10 PFU** *50% tissue culture infectious dose **plaque-forming units (1) Produced in chick embryo cells. (2) Produced in human diploid lung (WI-38) fibroblasts. (3) Produced in human diploid (MRC-5) cells. The vaccine may contain traces of recombinant human albumin (rHA). This vaccine contains a trace amount of neomycin. See section 4.3. Excipient(s) with known effect The vaccine contains 16 milligrams of sorbitol per dose. See section 4.4. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder and solvent for suspension for injection Before reconstitution, the powder is a white to pale yellow compact crystalline cake and the solvent is a clear colourless liquid. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications ProQuad is indicated for simultaneous vaccination against measles, mumps, rubella and varicella in individuals from 12 months of age. ProQuad can be administered to individuals from 9 months of age under special circumstances (e.g., to conform with national vaccination schedules, outbreak situations, or travel to a region with high prevalence of measles; see sections 4.2, 4.4, and 5.1). -

Summary of the WHO Position on Rubella Vaccine- July 2020 This
Summary of the WHO position on Rubella Vaccine- July 2020 This document replaces the WHO position paper on rubella vaccine published in the Weekly Epidemiological Record in July 2011. It incorporates the most recent developments in the field of rubella vaccines to provide updated guidance on the introduction and use of rubella-containing vaccines (RCVs) in national immunization schedules. It specifically updates guidance on co- administration of rubella vaccine with yellow fever (YF) vaccine and updates data and the WHO position on the control and elimination of rubella. Background Rubella is of public health importance because of the teratogenic potential of infections acquired during pregnancy. Rubella virus is generally recognized as the most common infectious cause of birth defects, accounting for an estimated 100 000 infants born with congenital rubella syndrome (CRS) each year worldwide. Infection with rubella virus within 12 days of conception and early pregnancy (usually within the first 8–10 weeks) may result in miscarriage, fetal death or CRS. For the rest of the population, rubella is an acute, usually mild viral infection transmitted by respiratory droplets, which affects susceptible children and adults worldwide. A number of live, attenuated rubella vaccines are currently available. Most rubella vaccines are available in combination with other vaccine antigens such as measles, mumps and varicella (MR, MMR, MMRV, respectively) and also in a monovalent formulation. WHO position In light of the global burden of CRS, the proven efficacy, effectiveness and safety of RCVs and regional elimination goals, WHO recommends that countries introduce RCVs into their national immunization programmes. This recommendation includes the opportunity offered by accelerated measles elimination activities to achieve both goals. -

Vaccine Information for PARENTS and CAREGIVERS
NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES Division of the National Health Laboratory Service VACCINE INFOR MATION FOR PARENTS & CAREGIVERS First Edition November 2016 Editors-in-Chief Nkengafac Villyen Motaze (MD, MSc, PhD fellow), Melinda Suchard (MBBCh, FCPath (SA), MMed) Edited by: Cheryl Cohen, (MBBCh, FCPath (SA) Micro, DTM&H, MSc (Epi), Phd) Lee Baker, (Dip Pharm) Lucille Blumberg, (MBBCh, MMed (Micro) ID (SA) FFTM (RCPS, Glasgow) DTM&H DOH DCH) Published by: Ideas Wise and Wonderful (IWW) for National Institute for Communicable Diseases (NICD) First Edition: Copyright © 2016 Contributions by: Clement Adu-Gyamfi (BSc Hons, MSc), Jayendrie Thaver (BSc), Kerrigan McCarthy, (MBBCh, FCPath (SA), DTM&H, MPhil (Theol) Kirsten Redman (BSc Hons), Nishi Prabdial-Sing (PhD), Nonhlanhla Mbenenge (MBBCh, MMED) Philippa Hime (midwife), Vania Duxbury (BSc Hons), Wayne Howard (BSc Hons) Acknowledgments: Amayeza, Vaccine Information Centre (http://www.amayeza-info.co.za/) Centre for Communicable Diseases Fact Sheets (http://www.cdc.gov/vaccines/hcp/vis/) World Health Organization Fact Sheets (http://www.who.int/mediacentre/factsheets/en/) National Department of Health, South Africa (http://www.health.gov.za/) Disclaimer This book is intended as an educational tool only. Information may be subject to change as schedules or formulations are updated. Summarized and simplified information is presented in this booklet. For full prescribing information and contraindications for vaccinations, please consult individual package inserts. There has been -

AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

2021-22 School Year New York State Immunization Requirements for School Entrance/Attendance1
2021-22 School Year New York State Immunization Requirements for School Entrance/Attendance1 NOTES: Children in a prekindergarten setting should be age-appropriately immunized. The number of doses depends on the schedule recommended by the Advisory Committee on Immunization Practices (ACIP). Intervals between doses of vaccine should be in accordance with the ACIP-recommended immunization schedule for persons 0 through 18 years of age. Doses received before the minimum age or intervals are not valid and do not count toward the number of doses listed below. See footnotes for specific information foreach vaccine. Children who are enrolling in grade-less classes should meet the immunization requirements of the grades for which they are age equivalent. Dose requirements MUST be read with the footnotes of this schedule Prekindergarten Kindergarten and Grades Grades Grade Vaccines (Day Care, 1, 2, 3, 4 and 5 6, 7, 8, 9, 10 12 Head Start, and 11 Nursery or Pre-k) Diphtheria and Tetanus 5 doses toxoid-containing vaccine or 4 doses and Pertussis vaccine 4 doses if the 4th dose was received 3 doses (DTaP/DTP/Tdap/Td)2 at 4 years or older or 3 doses if 7 years or older and the series was started at 1 year or older Tetanus and Diphtheria toxoid-containing vaccine Not applicable 1 dose and Pertussis vaccine adolescent booster (Tdap)3 Polio vaccine (IPV/OPV)4 4 doses 3 doses or 3 doses if the 3rd dose was received at 4 years or older Measles, Mumps and 1 dose 2 doses Rubella vaccine (MMR)5 Hepatitis B vaccine6 3 doses 3 doses or 2 doses of adult hepatitis B vaccine (Recombivax) for children who received the doses at least 4 months apart between the ages of 11 through 15 years Varicella (Chickenpox) 1 dose 2 doses vaccine7 Meningococcal conjugate Grades 2 doses vaccine (MenACWY)8 7, 8, 9, 10 or 1 dose Not applicable and 11: if the dose 1 dose was received at 16 years or older Haemophilus influenzae type b conjugate vaccine 1 to 4 doses Not applicable (Hib)9 Pneumococcal Conjugate 1 to 4 doses Not applicable vaccine (PCV)10 Department of Health 1. -

2021 Medicare Vaccine Coverage Part B Vs Part D
CDPHP® Medicare Advantage Vaccine Coverage Guide Part B (Medical) vs. Part D (Pharmacy) Medicare Part B (Medical): Medicare Part D (Pharmacy): Vaccinations or inoculations Vaccinations or inoculations are included when the administration is (except influenza, pneumococcal, reasonable and necessary for the prevention of illness. and hepatitis B for members at risk) are excluded unless they are directly related to the treatment of an injury or direct exposure to a disease or condition. • Influenza Vaccine (Flu) • BCG Vaccine • Pneumococcal Vaccine • Diphtheria/Tetanus/Acellular Pertussis Vaccine (ADACEL, (Pneumovax, Prevnar 13) BOOSTRIX, DAPTACEL, INFANRIX) • Hepatitis B Vaccine • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus (Recombivax, Engerix-B) Vaccine (KINRIX, QUADRACEL) for members at moderate • Diphtheria/Tetanus Vaccine (DT, Td, TDVAX, TENIVAC) to high risk • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus Vac • Other vaccines when directly cine/ Haemophilus Influenzae Type B Conjugate Vaccine (PENTACEL) related to the treatment of an • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus injury or direct exposure to a Vaccine/Hepatitis B Vaccine (PEDIARIX) disease or condition, such as: • Haemophilus Influenzae Type B Conjugate Vaccine (ActHIB, PedvaxHIB, • Antivenom Sera Hiberix) • Diphtheria/Tetanus Vaccine • Hepatitis A Vaccine, Inactivated (VAQTA) (DT, Td, TDVAX, TENIVAC) • Hepatitis B Vaccine, Recombinant (ENGERIX-B, RECOMBIVAX HB) • Rabies Virus Vaccine for members at low risk (RabAvert, -

Vaccinations for Adults with Chronic Liver Disease Or Infection
Vaccinations for Adults with Chronic Liver Disease or Infection This table shows which vaccinations you should have to protect your health if you have chronic hepatitis B or C infection or chronic liver disease (e.g., cirrhosis). Make sure you and your healthcare provider keep your vaccinations up to date. Vaccine Do you need it? Hepatitis A Yes! Your chronic liver disease or infection puts you at risk for serious complications if you get infected with the (HepA) hepatitis A virus. If you’ve never been vaccinated against hepatitis A, you need 2 doses of this vaccine, usually spaced 6–18 months apart. Hepatitis B Yes! If you already have chronic hepatitis B infection, you won’t need hepatitis B vaccine. However, if you have (HepB) hepatitis C or other causes of chronic liver disease, you do need hepatitis B vaccine. The vaccine is given in 2 or 3 doses, depending on the brand. Ask your healthcare provider if you need screening blood tests for hepatitis B. Hib (Haemophilus Maybe. Some adults with certain high-risk conditions, for example, lack of a functioning spleen, need vaccination influenzae type b) with Hib. Talk to your healthcare provider to find out if you need this vaccine. Human Yes! You should get this vaccine if you are age 26 years or younger. Adults age 27 through 45 may also be vacci- papillomavirus nated against HPV after a discussion with their healthcare provider. The vaccine is usually given in 3 doses over a (HPV) 6-month period. Influenza Yes! You need a dose every fall (or winter) for your protection and for the protection of others around you. -

Vaccinations for Preteens and Teens, Age 11-19 Years
Vaccinations for Preteens and Teens, Age 11–19 Years Getting immunized is a lifelong, life-protecting job. Make sure you and your healthcare provider keep your immunizations up to date. Check to be sure you’ve had all the vaccinations you need. Vaccine Do you need it? Chickenpox Yes! If you haven’t been vaccinated and haven’t had chickenpox, you need 2 doses of this vaccine. (varicella; Var) Anybody who was vaccinated with only 1 dose should get a second dose. Hepatitis A Yes! If you haven’t been vaccinated, you need 2 doses of this vaccine. Anybody who was vaccinated (HepA) with only 1 dose should get a second dose at least 6–18 months later. Hepatitis B Yes! This vaccine is recommended for all people age 0–18 years. You need a hepatitis B vaccine series (HepB) if you have not already received it. Haemophilus influ- Maybe. If you haven’t been vaccinated against Hib and have a high-risk condition (such as a non- enzae type b (Hib) functioning spleen), you need this vaccine. Human Yes! All preteens need 2 doses of HPV vaccine at age 11 or 12. Older teens who haven't been vacci- papillomavirus nated will need 2 or 3 doses. This vaccine protects against HPV, a common cause of genital warts and (HPV) several types of cancer, including cervical cancer and cancer of the anus, penis, and throat. Influenza Yes! Everyone age 6 months and older needs annual influenza vaccination every fall or winter and (Flu) for the rest of their lives. -
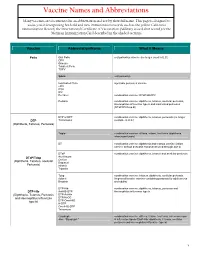
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus,