Adult Occupational Immunizations Massachusetts Recommendations and Requirements for 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Healthcare Personnel Vaccination Recommendations1 Vaccine Recommendations in Brief
Healthcare Personnel Vaccination Recommendations1 Vaccine Recommendations in brief Hepatitis B Give 3-dose series (dose #1 now, #2 in 1 month, #3 approximately 5 months after #2). Give IM. Obtain anti-HBs serologic testing 1–2 months after dose #3. Influenza Give 1 dose of influenza vaccine annually. Give inactivated injectable influenza vaccine intramuscularly or live attenuated influenza vaccine (LAIV) intranasally. MMR For healthcare personnel (HCP) born in 1957 or later without serologic evidence of immunity or prior vaccination, give 2 doses of MMR, 4 weeks apart. For HCP born prior to 1957, see below. Give SC. Varicella For HCP who have no serologic proof of immunity, prior vaccination, or history of varicella disease, (chickenpox) give 2 doses of varicella vaccine, 4 weeks apart. Give SC. Tetanus, diphtheria, Give a one-time dose of Tdap as soon as feasible to all HCP who have not received Tdap previously. pertussis Give Td boosters every 10 years thereafter. Give IM. Meningococcal Give 1 dose to microbiologists who are routinely exposed to isolates of N. meningitidis. Give IM or SC. Hepatitis A, typhoid, and polio vaccines are not routinely recommended for HCP who may have on-the-job exposure to fecal material. Hepatitis B Healthcare personnel (HCP) who perform tasks that may involve exposure to of live rubella vaccine). HCP with 2 documented doses of MMR are not blood or body fluids should receive a 3-dose series of hepatitis B vaccine at recommended to be serologically tested for immunity; but if they are tested 0-, 1-, and 6-month intervals. Test for hepatitis B surface antibody (anti-HBs) and results are negative or equivocal for measles, mumps, and/or rubella, to document immunity 1–2 months after dose #3. -

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

Safety of Immunization During Pregnancy a Review of the Evidence
Safety of Immunization during Pregnancy A review of the evidence Global Advisory Committee on Vaccine Safety © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

Mmrv Vaccine
VACCINE INFORMATION STATEMENT (Measles, Mumps, Many Vaccine Information Statements are available in Spanish and other languages. MMRV Vaccine Rubella and See www.immunize.org/vis Varicella) Hojas de información sobre vacunas están disponibles en español y en muchos otros What You Need to Know idiomas. Visite www.immunize.org/vis These are recommended ages. But children can get the Measles, Mumps, Rubella and second dose up through 12 years as long as it is at least 1 Varicella 3 months after the first dose. Measles, Mumps, Rubella, and Varicella (chickenpox) can be serious diseases: Children may also get these vaccines as 2 separate shots: MMR (measles, mumps and rubella) and Measles varicella vaccines. • Causes rash, cough, runny nose, eye irritation, fever. • Can lead to ear infection, pneumonia, seizures, brain 1 Shot (MMRV) or 2 Shots (MMR & Varicella)? damage, and death. • Both options give the same protection. Mumps • One less shot with MMRV. • Causes fever, headache, swollen glands. • Children who got the first dose as MMRV have • Can lead to deafness, meningitis (infection of the brain had more fevers and fever-related seizures (about and spinal cord covering), infection of the pancreas, 1 in 1,250) than children who got the first dose as painful swelling of the testicles or ovaries, and, rarely, separate shots of MMR and varicella vaccines on death. the same day (about 1 in 2,500). Rubella (German Measles) Your doctor can give you more information, • Causes rash and mild fever; and can cause arthritis, including the Vaccine Information Statements for (mostly in women). MMR and Varicella vaccines. -

(ACIP) General Best Guidance for Immunization
8. Altered Immunocompetence Updates This section incorporates general content from the Infectious Diseases Society of America policy statement, 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host (1), to which CDC provided input in November 2011. The evidence supporting this guidance is based on expert opinion and arrived at by consensus. General Principles Altered immunocompetence, a term often used synonymously with immunosuppression, immunodeficiency, and immunocompromise, can be classified as primary or secondary. Primary immunodeficiencies generally are inherited and include conditions defined by an inherent absence or quantitative deficiency of cellular, humoral, or both components that provide immunity. Examples include congenital immunodeficiency diseases such as X- linked agammaglobulinemia, SCID, and chronic granulomatous disease. Secondary immunodeficiency is acquired and is defined by loss or qualitative deficiency in cellular or humoral immune components that occurs as a result of a disease process or its therapy. Examples of secondary immunodeficiency include HIV infection, hematopoietic malignancies, treatment with radiation, and treatment with immunosuppressive drugs. The degree to which immunosuppressive drugs cause clinically significant immunodeficiency generally is dose related and varies by drug. Primary and secondary immunodeficiencies might include a combination of deficits in both cellular and humoral immunity. Certain conditions like asplenia and chronic renal disease also can cause altered immunocompetence. Determination of altered immunocompetence is important to the vaccine provider because incidence or severity of some vaccine-preventable diseases is higher in persons with altered immunocompetence; therefore, certain vaccines (e.g., inactivated influenza vaccine, pneumococcal vaccines) are recommended specifically for persons with these diseases (2,3). Administration of live vaccines might need to be deferred until immune function has improved. -

Adult Vaccines
What do flu, whooping cough, measles, shingles and pneumonia have in common? 1 They’re viruses that can make you very sick. 2 Vaccines can help prevent them. Protect yourself and those you care about. Get vaccinated at a network pharmacy near you. • Ask your pharmacist which vaccines are right for you. • Find out if your pharmacist can administer the recommended vaccinations. • Many vaccinations are covered by your plan at participating retail pharmacies. • Don’t forget to present your member ID card to the pharmacist at the time of service! The following vaccines are available and can be administered by pharmacists at participating network pharmacies: • Flu (seasonal influenza) • Meningitis • Travel Vaccines (typhoid, yellow • Tetanus/Diphtheria/Pertussis • Pneumonia fever, etc.) • Hepatitis • Rabies • Childhood Vaccines (MMR, etc.) • Human Papillomavirus (HPV) • Shingles/Zoster See other side for recommended adult vaccinations. The vaccinations you need ALL adults should get vaccinated for1: • Flu, every year. It’s especially important for pregnant women, older adults and people with chronic health conditions. • Tetanus, diphtheria and pertussis (whooping cough). Adults should get a one-time dose of the Tdap vaccine. It’s different from the tetanus vaccine (Td), which is given every 10 years. You may need additional vaccinations depending on your age1: Young adults not yet vaccinated need: Human papillomavirus (HPV) vaccine series (3 doses) if you are: • Female age 26 or younger • Male age 21 or younger • Male age 26 or younger who has sex with men, who is immunocompromised or who has HIV Adults born in the U.S. in 1957 or after need: Measles, mumps, rubella (MMR) vaccine2 Adults should get at least one dose of MMR vaccine, unless they’ve already gotten this vaccine or have immunity to measles, mumps and rubella Adults born in the U.S. -

Vaccines You Might Need?
Do You Know Which Adult Vaccines You Might Need? Vaccines are recommended for all adults based on factors such as age, travel, occupation, medical history, and vaccines they have had in the past. Below are the main vaccines you might need. But, this list may not include every vaccine that you need. Find out which vaccines you need by taking the quiz at: www.cdc.gov/vaccines/AdultQuiz/ ALL adults 19 and older, including pregnant women, need: Influenza vaccine every year • A flu vaccine is especially important for people with chronic health conditions, pregnant women, and older adults Tetanus, diphtheria, and pertussis (whooping cough) vaccine (Tdap) • Adults should get a one-time dose of Tdap. Adults can get Tdap no matter when they got their last tetanus vaccine (Td), which is given every 10 years • Pregnant women should get Tdap to protect themselves and their newborn babies from whooping cough In addition to influenza and Tdap vaccines, you may also need other vaccines depending on your age or other factors. Flip the page to learn more. Talk to your healthcare provider about which vaccines are right for you. National Center for Immunization and Respiratory Diseases Immunization Services Division CS235267 In addition to influenza and Tdap vaccines, you may also need other vaccines depending on your age… Young adults not yet vaccinated need: Adults born in the US in 1980 or after need: Human papillomavirus (HPV) vaccine series Varicella “chickenpox” vaccine* (3 doses) if you are: • Adults should get 2 doses of chickenpox vaccine • -

Vaccine Information for PARENTS and CAREGIVERS
NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES Division of the National Health Laboratory Service VACCINE INFOR MATION FOR PARENTS & CAREGIVERS First Edition November 2016 Editors-in-Chief Nkengafac Villyen Motaze (MD, MSc, PhD fellow), Melinda Suchard (MBBCh, FCPath (SA), MMed) Edited by: Cheryl Cohen, (MBBCh, FCPath (SA) Micro, DTM&H, MSc (Epi), Phd) Lee Baker, (Dip Pharm) Lucille Blumberg, (MBBCh, MMed (Micro) ID (SA) FFTM (RCPS, Glasgow) DTM&H DOH DCH) Published by: Ideas Wise and Wonderful (IWW) for National Institute for Communicable Diseases (NICD) First Edition: Copyright © 2016 Contributions by: Clement Adu-Gyamfi (BSc Hons, MSc), Jayendrie Thaver (BSc), Kerrigan McCarthy, (MBBCh, FCPath (SA), DTM&H, MPhil (Theol) Kirsten Redman (BSc Hons), Nishi Prabdial-Sing (PhD), Nonhlanhla Mbenenge (MBBCh, MMED) Philippa Hime (midwife), Vania Duxbury (BSc Hons), Wayne Howard (BSc Hons) Acknowledgments: Amayeza, Vaccine Information Centre (http://www.amayeza-info.co.za/) Centre for Communicable Diseases Fact Sheets (http://www.cdc.gov/vaccines/hcp/vis/) World Health Organization Fact Sheets (http://www.who.int/mediacentre/factsheets/en/) National Department of Health, South Africa (http://www.health.gov.za/) Disclaimer This book is intended as an educational tool only. Information may be subject to change as schedules or formulations are updated. Summarized and simplified information is presented in this booklet. For full prescribing information and contraindications for vaccinations, please consult individual package inserts. There has been -

The Safety of Influenza Vaccines in Children
Vaccine 33 (2015) F1–F67 Contents lists available at ScienceDirect Vaccine j ournal homepage: www.elsevier.com/locate/vaccine Review The safety of influenza vaccines in children: An Institute for Vaccine ଝ,ଝଝ Safety white paper a,b,∗ b,c d a Neal A. Halsey , Kawsar R. Talaat , Adena Greenbaum , Eric Mensah , a,b a,b a,b Matthew Z. Dudley , Tina Proveaux , Daniel A. Salmon a Department of International Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States b Institute for Vaccine Safety, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States c Center for Immunization Research, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States d Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, MD, United States a r t i c l e i n f o a b s t r a c t Keywords: Most influenza vaccines are generally safe, but influenza vaccines can cause rare serious adverse events. Influenza Some adverse events, such as fever and febrile seizures, are more common in children than adults. There Influenza vaccine can be differences in the safety of vaccines in different populations due to underlying differences in Vaccine safety genetic predisposition to the adverse event. Live attenuated vaccines have not been studied adequately Local reactions following IIV in children under 2 years of age to determine the risks of adverse events; more studies are needed to Cellulitis-like reactions address this and several other priority safety issues with all influenza vaccines in children. -
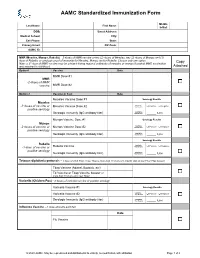
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Varicella (Chickenpox): Questions and Answers Q&A Information About the Disease and Vaccines
Varicella (Chickenpox): Questions and Answers Q&A information about the disease and vaccines What causes chickenpox? more common in infants, adults, and people with Chickenpox is caused by a virus, the varicella-zoster weakened immune systems. virus. How do I know if my child has chickenpox? How does chickenpox spread? Usually chickenpox can be diagnosed by disease his- Chickenpox spreads from person to person by direct tory and appearance alone. Adults who need to contact or through the air by coughing or sneezing. know if they’ve had chickenpox in the past can have It is highly contagious. It can also be spread through this determined by a laboratory test. Chickenpox is direct contact with the fluid from a blister of a per- much less common now than it was before a vaccine son infected with chickenpox, or from direct contact became available, so parents, doctors, and nurses with a sore from a person with shingles. are less familiar with it. It may be necessary to perform laboratory testing for children to confirm chickenpox. How long does it take to show signs of chickenpox after being exposed? How long is a person with chickenpox contagious? It takes from 10 to 21 days to develop symptoms after Patients with chickenpox are contagious for 1–2 days being exposed to a person infected with chickenpox. before the rash appears and continue to be conta- The usual time period is 14–16 days. gious through the first 4–5 days or until all the blisters are crusted over. What are the symptoms of chickenpox? Is there a treatment for chickenpox? The most common symptoms of chickenpox are rash, fever, coughing, fussiness, headache, and loss of appe- Most cases of chickenpox in otherwise healthy children tite.