Genetic Investigation of Patients with Tall Stature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tall Stature: a Difficult Diagnosis? Cristina Meazza1* , Chiara Gertosio2, Roberta Giacchero3, Sara Pagani1 and Mauro Bozzola1
Meazza et al. Italian Journal of Pediatrics (2017) 43:66 DOI 10.1186/s13052-017-0385-5 REVIEW Open Access Tall stature: a difficult diagnosis? Cristina Meazza1* , Chiara Gertosio2, Roberta Giacchero3, Sara Pagani1 and Mauro Bozzola1 Abstract Referral for an assessment of tall stature is less common than for short stature. Tall stature is defined as a height more than two standard deviations above the mean for age. The majority of subjects with tall stature show a familial tall stature or a constitutional advance of growth (CAG), which is a diagnosis of exclusion. After a careful physical evaluation, tall subjects may be divided into two groups: tall subjects with normal appearance and tall subjects with abnormal appearance. In the case of normal appearance, the paediatric endocrinologist will have to evaluate the growth rate. If it is normal for age and sex, the subject may be classified as having familial tall stature, CAG or obese subject, while if the growth rate is increased it is essential to evaluate pubertal status and thyroid status. Tall subjects with abnormal appearance and dysmorphisms can be classified into those with proportionate and disproportionate syndromes. A careful physical examination and an evaluation of growth pattern are required before starting further investigations. Physicians should always search for a pathological cause of tall stature, although the majority of children are healthy and they generally do not need treatment to cease growth progression. The most accepted and effective treatment for an excessive height prediction is inducing puberty early and leading to a complete fusion of the epiphyses and achievement of final height, using testosterone in males and oestrogens in females. -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

Level Estimates of Maternal Smoking and Nicotine Replacement Therapy During Pregnancy
Using primary care data to assess population- level estimates of maternal smoking and nicotine replacement therapy during pregnancy Nafeesa Nooruddin Dhalwani BSc MSc Thesis submitted to the University of Nottingham for the degree of Doctor of Philosophy November 2014 ABSTRACT Background: Smoking in pregnancy is the most significant preventable cause of poor health outcomes for women and their babies and, therefore, is a major public health concern. In the UK there is a wide range of interventions and support for pregnant women who want to quit. One of these is nicotine replacement therapy (NRT) which has been widely available for retail purchase and prescribing to pregnant women since 2005. However, measures of NRT prescribing in pregnant women are scarce. These measures are vital to assess its usefulness in smoking cessation during pregnancy at a population level. Furthermore, evidence of NRT safety in pregnancy for the mother and child’s health so far is nebulous, with existing studies being small or using retrospectively reported exposures. Aims and Objectives: The main aim of this work was to assess population- level estimates of maternal smoking and NRT prescribing in pregnancy and the safety of NRT for both the mother and the child in the UK. Currently, the only population-level data on UK maternal smoking are from repeated cross-sectional surveys or routinely collected maternity data during pregnancy or at delivery. These obtain information at one point in time, and there are no population-level data on NRT use available. As a novel approach, therefore, this thesis used the routinely collected primary care data that are currently available for approximately 6% of the UK population and provide longitudinal/prospectively recorded information throughout pregnancy. -
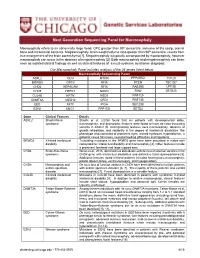
Macrocephaly Information Sheet 6-13-19
Next Generation Sequencing Panel for Macrocephaly Clinical Features: Macrocephaly refers to an abnormally large head, OFC greater than 98th percentile, inclusive of the scalp, cranial bone and intracranial contents. Megalencephaly, brain weight/volume ratio greater than 98th percentile, results from true enlargement of the brain parenchyma [1]. Megalencephaly is typically accompanied by macrocephaly, however macrocephaly can occur in the absence of megalencephaly [2]. Both macrocephaly and megalencephaly can been seen as isolated clinical findings as well as clinical features of a mutli-systemic syndromic diagnosis. Our Macrocephaly Panel includes analysis of the 36 genes listed below. Macrocephaly Sequencing Panel ASXL2 GLI3 MTOR PPP2R5D TCF20 BRWD3 GPC3 NFIA PTEN TBC1D7 CHD4 HEPACAM NFIX RAB39B UPF3B CHD8 HERC1 NONO RIN2 ZBTB20 CUL4B KPTN NSD1 RNF125 DNMT3A MED12 OFD1 RNF135 EED MITF PIGA SEC23B EZH2 MLC1 PPP1CB SETD2 Gene Clinical Features Details ASXL2 Shashi-Pena Shashi et al. (2016) found that six patients with developmental delay, syndrome macrocephaly, and dysmorphic features were found to have de novo truncating variants in ASXL2 [3]. Distinguishing features were macrocephaly, absence of growth retardation, and variability in the degree of intellectual disabilities The phenotype also consisted of prominent eyes, arched eyebrows, hypertelorism, a glabellar nevus flammeus, neonatal feeding difficulties and hypotonia. BRWD3 X-linked intellectual Truncating mutations in the BRWD3 gene have been described in males with disability nonsyndromic intellectual disability and macrocephaly [4]. Other features include a prominent forehead and large cupped ears. CHD4 Sifrim-Hitz-Weiss Weiss et al., 2016, identified five individuals with de novo missense variants in the syndrome CHD4 gene with intellectual disabilities and distinctive facial dysmorphisms [5]. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -

Sotos Syndrome
European Journal of Human Genetics (2007) 15, 264–271 & 2007 Nature Publishing Group All rights reserved 1018-4813/07 $30.00 www.nature.com/ejhg PRACTICAL GENETICS In association with Sotos syndrome Sotos syndrome is an autosomal dominant condition characterised by a distinctive facial appearance, learning disability and overgrowth resulting in tall stature and macrocephaly. In 2002, Sotos syndrome was shown to be caused by mutations and deletions of NSD1, which encodes a histone methyltransferase implicated in chromatin regulation. More recently, the NSD1 mutational spectrum has been defined, the phenotype of Sotos syndrome clarified and diagnostic and management guidelines developed. Introduction In brief Sotos syndrome was first described in 1964 by Juan Sotos Sotos syndrome is characterised by a distinctive facial and the major diagnostic criteria of a distinctive facial appearance, learning disability and childhood over- appearance, childhood overgrowth and learning disability growth. were established in 1994 by Cole and Hughes.1,2 In 2002, Sotos syndrome is associated with cardiac anomalies, cloning of the breakpoints of a de novo t(5;8)(q35;q24.1) renal anomalies, seizures and/or scoliosis in B25% of translocation in a child with Sotos syndrome led to the cases and a broad variety of additional features occur discovery that Sotos syndrome is caused by haploinsuffi- less frequently. ciency of the Nuclear receptor Set Domain containing NSD1 abnormalities, such as truncating mutations, protein 1 gene, NSD1.3 Subsequently, extensive analyses of missense mutations in functional domains, partial overgrowth cases have shown that intragenic NSD1 muta- gene deletions and 5q35 microdeletions encompass- tions and 5q35 microdeletions encompassing NSD1 cause ing NSD1, are identifiable in the majority (490%) of 490% of Sotos syndrome cases.4–10 In addition, NSD1 Sotos syndrome cases. -

Soonerstart Automatic Qualifying Syndromes and Conditions
SoonerStart Automatic Qualifying Syndromes and Conditions - Appendix O Abetalipoproteinemia Acanthocytosis (see Abetalipoproteinemia) Accutane, Fetal Effects of (see Fetal Retinoid Syndrome) Acidemia, 2-Oxoglutaric Acidemia, Glutaric I Acidemia, Isovaleric Acidemia, Methylmalonic Acidemia, Propionic Aciduria, 3-Methylglutaconic Type II Aciduria, Argininosuccinic Acoustic-Cervico-Oculo Syndrome (see Cervico-Oculo-Acoustic Syndrome) Acrocephalopolysyndactyly Type II Acrocephalosyndactyly Type I Acrodysostosis Acrofacial Dysostosis, Nager Type Adams-Oliver Syndrome (see Limb and Scalp Defects, Adams-Oliver Type) Adrenoleukodystrophy, Neonatal (see Cerebro-Hepato-Renal Syndrome) Aglossia Congenita (see Hypoglossia-Hypodactylia) Aicardi Syndrome AIDS Infection (see Fetal Acquired Immune Deficiency Syndrome) Alaninuria (see Pyruvate Dehydrogenase Deficiency) Albers-Schonberg Disease (see Osteopetrosis, Malignant Recessive) Albinism, Ocular (includes Autosomal Recessive Type) Albinism, Oculocutaneous, Brown Type (Type IV) Albinism, Oculocutaneous, Tyrosinase Negative (Type IA) Albinism, Oculocutaneous, Tyrosinase Positive (Type II) Albinism, Oculocutaneous, Yellow Mutant (Type IB) Albinism-Black Locks-Deafness Albright Hereditary Osteodystrophy (see Parathyroid Hormone Resistance) Alexander Disease Alopecia - Mental Retardation Alpers Disease Alpha 1,4 - Glucosidase Deficiency (see Glycogenosis, Type IIA) Alpha-L-Fucosidase Deficiency (see Fucosidosis) Alport Syndrome (see Nephritis-Deafness, Hereditary Type) Amaurosis (see Blindness) Amaurosis -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008. -

With a Learning Disability; Guidance for General Practice
Improving identification of people with a learning disability: guidance for general practice NHS England and Improvement Publishing Approval Reference: 001030 Version 1 NHS England and NHS Improvement Contents Introduction .................................................................................... 2 Actions for practices ....................................................................... 4 Appendix 1: List of codes that indicate a learning disability ........... 7 Appendix 2: List of codes that may indicate a learning disability . 14 Appendix 3: List of outdated codes .............................................. 20 Appendix 4: Learning disability identification check-list ............... 22 1 | Contents Introduction 1. The NHS Long Term Plan1 commits to improve uptake of the existing annual health check in primary care for people aged over 14 years with a learning disability, so that at least 75% of those eligible have a learning disability health check each year. 2. There is also a need to increase the number of people receiving the annual seasonal flu vaccination, given the level of avoidable mortality associated with respiratory problems. 3. In 2017/18, only 44.6% of patients with a learning disability received a flu vaccination and only 55.1% of patients with a learning disability received an annual learning disability health check.2 4. In June 2019, NHS England and NHS Improvement announced a series of measures to improve coverage of annual health checks and flu vaccination for people with a learning disability. One of the commitments was to improve the quality of registers for people with a learning disability3. Clinical coding review 5. Most GP practices have developed a register of their patients known to have a learning disability. This has been developed from clinical diagnoses, from information gathered from learning disabilities teams and social services and has formed the basis of registers for people with learning disability developed for the Quality and Outcomes Framework (QOF). -

11 Selected Syndromes and Associations
11 Selected Syndromes and Associations ĭ Apert Syndrome Definition: Also known as acrocephalic syndac- Other anomalies of the cardiac, renal, and tyly syndrome. It is characterized by a “tower- gastrointestinal systems may also be present. shaped” head, facial dysmorphism, and sym- metrical syndactyly of the fingers and toes. Ultrasound findings: The earliest prenatal diag- nosis was possible at 12 weeks of gestation with Incidence: about one in 100 000 births. nuchal translucency measurements. Syndactyly First described in 1906 by Apert. could also be detected at this stage. At a later stage, an unusual shape of the head resulting Etiology/genetics: Partly autosomal-dominant from premature closure of the cranial sutures inheritance, but frequently sporadic occurrence (tower-shaped head) and facial dysmorphism (new mutation). Advanced paternal age is a fac- (hypoplasia of the midfacial region) are charac- tor favoring its occurrence. Gene defect in the fi- teristic features. Detailed scanning may also re- broblast growth factor receptor-2 gene (FGFR2), veal deep-set ears, unusual shape of the nose, gene locus: 10q26. exophthalmos, and hypertelorism. Clinical features: “Tower-shaped” head, early Differential diagnosis: Carpenter syndrome, closure of cranial sutures, anomalies of the cervi- Crouzon syndrome, cloverleaf skull anomaly, cal vertebral column. Facial anomalies: denting Pfeiffer syndrome, thanatophoric dysplasia, of the forehead in the supraorbital region, hyper- achondrogenesis, Cornelia de Lange syndrome, telorism, flat orbital bone, exophthalmos, squint, EEC syndrome, Fryns syndrome, Joubert syn- deep-set ears, small, beak-shaped nose, syndac- drome, multiple pterygium syndrome, Noonan tyly (as extreme as “spoon hands”), fusion of the syndrome, Roberts syndrome, Smith–Lemli– bony parts of fingers II–IV, short fingers, possibly Opitz syndrome, trisomy 21. -

Essential Genetics 5
Essential genetics 5 Disease map on chromosomes 例 Gaucher disease 単一遺伝子病 天使病院 Prader-Willi syndrome 隣接遺伝子症候群,欠失が主因となる疾患 臨床遺伝診療室 外木秀文 Trisomy 13 複数の遺伝子の重複によって起こる疾患 挿画 Koromo 遺伝子の座位あるいは欠失等の範囲を示す Copyright (c) 2010 Social Medical Corporation BOKOI All Rights Reserved. Disease map on chromosome 1 Gaucher disease Chromosome 1q21.1 1p36 deletion syndrome deletion syndrome Adrenoleukodystrophy, neonatal Cardiomyopathy, dilated, 1A Zellweger syndrome Charcot-Marie-Tooth disease Emery-Dreifuss muscular Hypercholesterolemia, familial dystrophy Hutchinson-Gilford progeria Ehlers-Danlos syndrome, type VI Muscular dystrophy, limb-girdle type Congenital disorder of Insensitivity to pain, congenital, glycosylation, type Ic with anhidrosis Diamond-Blackfan anemia 6 Charcot-Marie-Tooth disease Dejerine-Sottas syndrome Marshall syndrome Stickler syndrome, type II Chronic granulomatous disease due to deficiency of NCF-2 Alagille syndrome 2 Copyright (c) 2010 Social Medical Corporation BOKOI All Rights Reserved. Disease map on chromosome 2 Epiphyseal dysplasia, multiple Spondyloepimetaphyseal dysplasia Brachydactyly, type D-E, Noonan syndrome Brachydactyly-syndactyly syndrome Peters anomaly Synpolydactyly, type II and V Parkinson disease, familial Leigh syndrome Seizures, benign familial Multiple pterygium syndrome neonatal-infantile Escobar syndrome Ehlers-Danlos syndrome, Brachydactyly, type A1 type I, III, IV Waardenburg syndrome Rhizomelic chondrodysplasia punctata, type 3 Alport syndrome, autosomal recessive Split-hand/foot malformation Crigler-Najjar -

Craniosynostosis Precision Panel Overview Indications Clinical Utility
Craniosynostosis Precision Panel Overview Craniosynostosis is defined as the premature fusion of one or more cranial sutures, often resulting in abnormal head shape. It is a developmental craniofacial anomaly resulting from a primary defect of ossification (primary craniosynostosis) or, more commonly, from a failure of brain growth (secondary craniosynostosis). As well, craniosynostosis can be simple when only one suture fuses prematurely or complex/compound when there is a premature fusion of multiple sutures. Complex craniosynostosis are usually associated with other body deformities. The main morbidity risk is the elevated intracranial pressure and subsequent brain damage. When left untreated, craniosynostosis can cause serious complications such as developmental delay, facial abnormality, sensory, respiratory and neurological dysfunction, eye anomalies and psychosocial disturbances. In approximately 85% of the cases, this disease is isolated and nonsyndromic. Syndromic craniosynostosis usually present with multiorgan complications. The Igenomix Craniosynostosis Precision Panel can be used to make a directed and accurate diagnosis ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes involved. Indications The Igenomix Craniosynostosis Precision Panel is indicated for those patients with a clinical diagnosis or suspicion with or without the following manifestations: ‐ Microcephaly ‐ Scaphocephaly (elongated head) ‐ Anterior plagiocephaly ‐ Brachycephaly ‐ Torticollis ‐ Frontal bossing Clinical Utility The clinical utility of this panel is: - The genetic and molecular confirmation for an accurate clinical diagnosis of a symptomatic patient. - Early initiation of treatment in the form surgical procedures to relieve fused sutures, midface advancement, limited phase of orthodontic treatment and combined 1 orthodontics/orthognathic surgery treatment.