Evidence for Flower Mediated Assembly in Spring Ephemeral Understory Communities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydrastis Canadensis L.) in Pennsylvania: Explaining and Predicting Species Distribution in a Northern Edge of Range State
bioRxiv preprint doi: https://doi.org/10.1101/694802; this version posted July 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title: Associated habitat and suitability modeling of goldenseal (Hydrastis canadensis L.) in Pennsylvania: explaining and predicting species distribution in a northern edge of range state. *1Grady H. Zuiderveen, 1Xin Chen, 1,2Eric P. Burkhart, 1,3Douglas A. Miller 1Department of Ecosystem Science and Management, Pennsylvania State University, University Park, PA 16802 2Shavers Creek Environmental Center, 3400 Discovery Rd, Petersburg, PA 16669 3Department of Geography, Pennsylvania State University, University Park, PA 16802 *telephone: (616) 822-8685; email: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/694802; this version posted July 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Goldenseal (Hydrastis canadensis L.) is a well-known perennial herb indigenous to forested areas in eastern North America. Owing to conservation concerns including wild harvesting for medicinal markets, habitat loss and degradation, and an overall patchy and often inexplicable absence in many regions, there is a need to better understand habitat factors that help determine the presence and distribution of goldenseal populations. In this study, flora and edaphic factors associated with goldenseal populations throughout Pennsylvania—a state near the northern edge of its range—were documented and analyzed to identify habitat indicators and provide possible in situ stewardship and farming (especially forest-based farming) guidance. -

Flora of New Jersey Project Approved Nomenclature
FLORA OF NEW JERSEY PROJECT APPROVED NOMENCLATURE FNJP Authority Name: William Olson Date: March 14, 2010 Violaceae – Violet Family New Jersey Violet Family (synonyms as indented) Hybanthus Jacq. Hybanthus concolor (T.F. Forst.) Spreng. Eastern Green-Violet Syn. – Cubelium concolor (T.F. Forst.) Raf. Syn. – Viola concolor T.F. Forst. Viola L. Viola affinis Le Conte - Sand Violet, Le Conte’s Violet Syn. – Viola chalcosperma Brainerd Syn. – Viola rosacea Brainerd Syn. – Viola sororia ssp. affinis (Le Conte) R.J. Little Syn. – Viola sororia var. affinis (Le Conte) McKinney Viola arvensis Murr. - European Field Pansy EXOTIC Syn. – Viola tricolor var. arvensis (Murr.) Boiss. Viola bicolor Pursh - Field Pansy EXOTIC Syn. – Viola kitaibeliana var. rafinesquei Fern. Syn. – Viola kitaibeliana auct. non J.A. Schultes Syn. – Viola rafinesquei Greene Viola blanda Willd. - Sweet White Violet var. blanda var. palustriformis Gray RARE Syn. – Viola incognita Brainerd Syn. – Viola incognita var. forbesii Brainerd THE FLORA OF NEW JERSEY PROJECT IS A VOLUNTEER EFFORT AIMED AT THE PRODUCTION OF A MANUAL TO THE VASCULAR FLORA OF NEW JERSEY. Viola brittoniana Pollard - Northern Coastal Violet RARE var. brittoniana RARE Syn. – Viola pedatifida ssp. brittoniana (Pollard) McKinney var. pectinata (Bickn.) Alexander RARE Syn. – Viola pectinata Bickn. Viola canadensis L. - Canadian White Violet, Canada Violet var. canadensis RARE Syn. – Viola canadensis var. corymbosa Nutt. ex Torr. & Gray Viola cucullata Ait. - Marsh Blue Violet, Marsh Violet Syn. – Viola cucullata var. microtitis Brainerd Syn. – Viola obliqua Hill Viola hirsutula Brainerd - Southern Woodland Violet RARE Viola labradorica Schrank - Alpine Violet, American Dog Violet Syn. – Viola adunca var. minor (Hook.) Fern. Syn. – Viola conspersa Reinchenb. -

Dispersal Effects on Species Distribution and Diversity Across Multiple Scales in the Southern Appalachian Mixed Mesophytic Flora
DISPERSAL EFFECTS ON SPECIES DISTRIBUTION AND DIVERSITY ACROSS MULTIPLE SCALES IN THE SOUTHERN APPALACHIAN MIXED MESOPHYTIC FLORA Samantha M. Tessel A dissertation submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Ecology in the Curriculum for the Environment and Ecology. Chapel Hill 2017 Approved by: Peter S. White Robert K. Peet Alan S. Weakley Allen H. Hurlbert Dean L. Urban ©2017 Samantha M. Tessel ALL RIGHTS RESERVED ii ABSTRACT Samantha M. Tessel: Dispersal effects on species distribution and diversity across multiple scales in the southern Appalachian mixed mesophytic flora (Under the direction of Peter S. White) Seed and spore dispersal play important roles in the spatial distribution of plant species and communities. Though dispersal processes are often thought to be more important at larger spatial scales, the distribution patterns of species and plant communities even at small scales can be determined, at least in part, by dispersal. I studied the influence of dispersal in southern Appalachian mixed mesophytic forests by categorizing species by dispersal morphology and by using spatial pattern and habitat connectivity as predictors of species distribution and community composition. All vascular plant species were recorded at three nested sample scales (10000, 1000, and 100 m2), on plots with varying levels of habitat connectivity across the Great Smoky Mountains National Park. Models predicting species distributions generally had higher predictive power when incorporating spatial pattern and connectivity, particularly at small scales. Despite wide variation in performance, models of locally dispersing species (species without adaptations to dispersal by wind or vertebrates) were most frequently improved by the addition of spatial predictors. -

Checklist Flora of the Former Carden Township, City of Kawartha Lakes, on 2016
Hairy Beardtongue (Penstemon hirsutus) Checklist Flora of the Former Carden Township, City of Kawartha Lakes, ON 2016 Compiled by Dale Leadbeater and Anne Barbour © 2016 Leadbeater and Barbour All Rights reserved. No part of this publication may be reproduced, stored in a retrieval system or database, or transmitted in any form or by any means, including photocopying, without written permission of the authors. Produced with financial assistance from The Couchiching Conservancy. The City of Kawartha Lakes Flora Project is sponsored by the Kawartha Field Naturalists based in Fenelon Falls, Ontario. In 2008, information about plants in CKL was scattered and scarce. At the urging of Michael Oldham, Biologist at the Natural Heritage Information Centre at the Ontario Ministry of Natural Resources and Forestry, Dale Leadbeater and Anne Barbour formed a committee with goals to: • Generate a list of species found in CKL and their distribution, vouchered by specimens to be housed at the Royal Ontario Museum in Toronto, making them available for future study by the scientific community; • Improve understanding of natural heritage systems in the CKL; • Provide insight into changes in the local plant communities as a result of pressures from introduced species, climate change and population growth; and, • Publish the findings of the project . Over eight years, more than 200 volunteers and landowners collected almost 2000 voucher specimens, with the permission of landowners. Over 10,000 observations and literature records have been databased. The project has documented 150 new species of which 60 are introduced, 90 are native and one species that had never been reported in Ontario to date. -

Chapter 4 Native Plants for Landscape Use in Kentucky
Chapter 4 Native Plants for Landscape Use In Kentucky A publication of the Louisville Water Company Wellhead Protection Plan, Phase III Source Reduction Grant # X9-96479407-0 Chapter 4 Native Plants for Landscape Use in Kentucky Native Wildflowers and Ferns The U. S. Department of Transportation, (US DOT), has developed a listing of native plants, (ferns, annuals, perennials, shrubs, and trees), that may be used in landscaping in the State of Kentucky. Other agencies have also developed listings of native plants, which have been integrated into the list within this guidebook. While this list is, by no means, a complete report of the native species that may be found in Kentucky, it offers a starting point for additional research, should the homeowner wish to find additional KY native plants for use in a landscape design, or to check if a plant is native to the State. A reference book titled Wildflowers and Ferns of Kentucky, which was recommended by personnel at the Salato Wildlife Center as an excellent reference for native plants, was also used to develop the list. (A full bibliography is listed at the end of this chapter.) While many horticultural and botanical experts may dispute the inclusion of specific plants on the listing, or wish to add more plants, the list represents the latest information available for research, by the amateur, at the time. The information listed within the list was taken at face value, and no judgment calls were made about the suitability of plants for the list. The author makes no claims as to the completeness, accuracy, or timeliness of this list. -

Field Checklist
14 September 2020 Cystopteridaceae (Bladder Ferns) __ Cystopteris bulbifera Bulblet Bladder Fern FIELD CHECKLIST OF VASCULAR PLANTS OF THE KOFFLER SCIENTIFIC __ Cystopteris fragilis Fragile Fern RESERVE AT JOKERS HILL __ Gymnocarpium dryopteris CoMMon Oak Fern King Township, Regional Municipality of York, Ontario (second edition) Aspleniaceae (Spleenworts) __ Asplenium platyneuron Ebony Spleenwort Tubba Babar, C. Sean Blaney, and Peter M. Kotanen* Onocleaceae (SensitiVe Ferns) 1Department of Ecology & Evolutionary Biology 2Atlantic Canada Conservation Data __ Matteuccia struthiopteris Ostrich Fern University of Toronto Mississauga Centre, P.O. Box 6416, Sackville NB, __ Onoclea sensibilis SensitiVe Fern 3359 Mississauga Road, Mississauga, ON Canada E4L 1G6 Canada L5L 1C6 Athyriaceae (Lady Ferns) __ Deparia acrostichoides SilVery Spleenwort *Correspondence author. e-mail: [email protected] Thelypteridaceae (Marsh Ferns) The first edition of this list Was compiled by C. Sean Blaney and Was published as an __ Parathelypteris noveboracensis New York Fern appendix to his M.Sc. thesis (Blaney C.S. 1999. Seed bank dynamics of native and exotic __ Phegopteris connectilis Northern Beech Fern plants in open uplands of southern Ontario. University of Toronto. __ Thelypteris palustris Marsh Fern https://tspace.library.utoronto.ca/handle/1807/14382/). It subsequently Was formatted for the web by P.M. Kotanen and made available on the Koffler Scientific Reserve Website Dryopteridaceae (Wood Ferns) (http://ksr.utoronto.ca/), Where it Was revised periodically to reflect additions and taxonomic __ Athyrium filix-femina CoMMon Lady Fern changes. This second edition represents a major revision reflecting recent phylogenetic __ Dryopteris ×boottii Boott's Wood Fern and nomenclatural changes and adding additional species; it will be updated periodically. -

The Vascular Flora of the Natchez Trace Parkway
THE VASCULAR FLORA OF THE NATCHEZ TRACE PARKWAY (Franklin, Tennessee to Natchez, Mississippi) Results of a Floristic Inventory August 2004 - August 2006 © Dale A. Kruse, 2007 © Dale A. Kruse 2007 DATE SUBMITTED 28 February 2008 PRINCIPLE INVESTIGATORS Stephan L. Hatch Dale A. Kruse S. M. Tracy Herbarium (TAES), Texas A & M University 2138 TAMU, College Station, Texas 77843-2138 SUBMITTED TO Gulf Coast Inventory and Monitoring Network Lafayette, Louisiana CONTRACT NUMBER J2115040013 EXECUTIVE SUMMARY The “Natchez Trace” has played an important role in transportation, trade, and communication in the region since pre-historic times. As the development and use of steamboats along the Mississippi River increased, travel on the Trace diminished and the route began to be reclaimed by nature. A renewed interest in the Trace began during, and following, the Great Depression. In the early 1930’s, then Mississippi congressman T. J. Busby promoted interest in the Trace from a historical perspective and also as an opportunity for employment in the area. Legislation was introduced by Busby to conduct a survey of the Trace and in 1936 actual construction of the modern roadway began. Development of the present Natchez Trace Parkway (NATR) which follows portions of the original route has continued since that time. The last segment of the NATR was completed in 2005. The federal lands that comprise the modern route total about 52,000 acres in 25 counties through the states of Alabama, Mississippi, and Tennessee. The route, about 445 miles long, is a manicured parkway with numerous associated rest stops, parks, and monuments. Current land use along the NATR includes upland forest, mesic prairie, wetland prairie, forested wetlands, interspersed with numerous small agricultural croplands. -

The Violets of Ohio
56 The Ohio Naturalist. [Vol. XIII, No. 3, THE VIOLETS OF OHIO. ROSE GORMLEY. The following list includes all of the violets known to occur in Ohio. It is probable, however, that a number of others occur in the state. The distribution given is based on material in the Ohio State Herbarium. In this list an attempt has been made to arrange the species in true phyletic series, the least specialized in each group standing at the beginning and the most highly specialized at the end. Violaceae. Small herbs, with bisporangiate, hypogynous, zygomorphic, axillary, nodding flowers and alternate, simple or lobed stipulate leaves. Sepals, petals and stamens 5 each; anthers erect, introrse, connivant or synantherous; ovulary of 3 carpels, unilocular with 3 parietal placentas; lower petal enlarged usually with a spur; fruit a loculicidal capsule; seeds anatropous, with endosperm, embryo straight. 1. Sepals not auricled, stamens united, petals nearly equal. Cubelium. 1. Sepals more or less auricled at the base, stamens distinct, lower petal spurred. Viola. Cubelium. Perennial, erect, leafy stemmed herb, the leaves, entire or obscurely dentate; small greenish flowers, one to three together in the axils, petals nearly equal, the lower somewhat gibbous; anthers sessile, completely united into a sheath, glandular at the base. A monotypic genus of North America. Cubelium concolor. (Forst) Raf. Green Violet. Plants 1—2\ ft. high, hairy; leaves 2—5 in. long, entire, pointed at both ends. Auglaize, Belmont, Brown, Clermont, Fairfield, Franklin, Hamil- ton, Lake, Licking, Noble, Pike, Shelby, Warren Co. Viola. Herbs with aerial leafy stems, or geophilous stems; flowers solitary or rarely 2 in the axils, early flowers petaliferous, often sterile, usually succeeded by apetalous, cleistogamous flowers which produce abundant seed; the two lower stamens bearing spurs which project into spur of the odd petal; capsules, three valved, elastically dehiscent. -

Field Guides and Floras: • Magee, Dennis W
* THE SHORT ALDRICH TRAIL SPRING EPHEMERAL PLANTS Isabel Marlens Spring 2012 * 1 Introduction . .3 The Short Aldrich Trail . .4 Plant Profiles:* Claytonia caroliniana . 6 Sanguinaria canadensis . 7 Anemone acutiloba . 8 Caulophyllum thalictroides . 9 Dicentra cucullaria . 10 Cardamine spp . .11 Erythronium americanum . .12 Ranunculus abortivus . .13 Trillium erectum . 14 Viola spp. .. .15 Dicentra canadensis . .17 Trillium grandiflorum . 18 Tiarella cordifolia . 19 Arisaema triphyllum . .20 Mitella diphylla . .21 Asarum canadense . 22 Polygonatum biflorum . .23 End Note . .24 Sources . .24 *Because this field guide has a focus on first flowering dates, the plant profile order is based upon phenological characteristics. 2 INTRODUCTION Nature's first green is gold, Her hardest hue to hold. Her early leaf's a flower; But only so an hour. Then leaf subsides to leaf. So Eden sank to grief, So dawn goes down to day. Nothing gold can stay. – Robert Frost Robert Frost wrote this small poem about, among other things, death and spring ephemeral plants three years after he moved to a farmhouse in Shaftesbury, Vermont. The house is scarcely five miles, as the crow flies, from the Short Aldrich Trail in North Bennington. The poem is one of the most famous in a collection that won the Pulitzer Prize. It is not hard to see why. It reflects a fixation – a common one, perhaps, because it is so caught up with our perceptions of beauty, of grief, of human life and loss – with the pathos of precious things that seem to fade too fast. Spring ephemerals represent perfectly the notion of the exquisite that cannot last. -
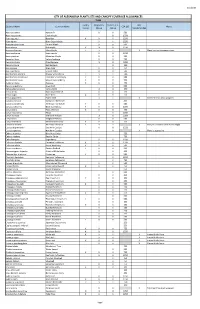
City of Alexandria Plant Lists and Canopy Coverage Allowances Trees
3/1/2019 CITY OF ALEXANDRIA PLANT LISTS AND CANOPY COVERAGE ALLOWANCES TREES Locally Regionally Eastern U.S. Not Botanical Name Common Name CCA (SF) Notes Native Native Native Recommended Abies balsamea Balsam Fir X X 500 Acer leucoderme Chalk Maple X 1250 Acer negundo Boxelder X X X 1250 Acer nigrum Black Sugar Maple X X 1250 Acer pensylvanicum Striped Maple X X 500 Acer rubrum Red maple X X X 1250 Acer saccharinum Silver Maple X X X X Plant has maintenance issues. Acer saccharum Sugar maple X X 1250 Acer spicatum Mountain Maple X X 500 Aesculus flava Yellow Buckeye X X 750 Aesculus glabra Ohio Buckeye X X 1250 Aesculus pavia Red Buckeye X 500 Alnus incana Gray Alder X X 750 Alnus maritima Seaside Alder X X 500 Amelanchier arborea Downy Serviceberry X X X 500 Amelanchier canadensis Canadian Serviceberry X X X 500 Amelanchier laevis Smooth Serviceberry X X X 750 Asimina triloba Pawpaw X X X 750 Betula populifolia Gray Birch X 500 Betula alleghaniensis Yellow Birch X X 750 Betula lenta Black (Sweet) birch X X 750 Betula nigra River birch X X X 750 Betula papyrifera Paper Birch X X X Northern/mountain adapted. Carpinua betulus European Hornbeam 250 Carpinus caroliniana American Hornbeam X X X 500 Carya cordiformis Bitternut Hickory X X X 1250 Carya glabra Pignut Hickory X X X 750 Carya illinoinensis Pecan X 1250 Carya laciniosa Shellbark Hickory X X 1250 Carya ovata Shagbark Hickory X X 500 Carya tomentosa Mockernut Hickory X X X 750 Castanea dentata American Chestnut X X X X Not yet recommended due to Blight Catalpa bignonioides Southern Catalpa X X 1250 Catalpa speciosa Northern Catalpa X X Plant is aggressive. -

Massachusetts 2012 Final State Wetland Plant List
MASSACHUSETTS 2012 FINAL STATE WETLAND PLANT LIST Lichvar, R.W. 2012. The National Wetland Plant List. ERDC/CRREL TR‐12‐11. Hanover, NH: U.S. Army Corps of Engineers, Cold Regions Research and Engineering Laboratory. http://acwc.sdp.sirsi.net/client/search/asset:asset?t:ac=$N/1012381 User Notes: 1) Plant species not listed are considered UPL for wetland delineation purposes. 2) A few UPL species are listed because they are rated FACU or wetter in at least one Corps region. 3) Some state boundaries lie within two or more Corps Regions. If a species occurs in one of the regions but not the other, its rating will be shown in one column and the other column will show a dash. For example, there are two Corps regions in North Dakota, the GP and the MW. Agrostis exarata occurs in the GP but not the MW, so it is listed as: Species Authorship GP MW Common Name Agrostis exarata Trin. FACW ‐ Spiked Bent Species Authorship NCNE Common Name Abies balsamea (L.) P. Mill. FAC Balsam Fir Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. UPL White Fir Abutilon theophrasti Medik. FACU Velvetleaf Acalypha gracilens Gray FACU Slender Three‐Seed‐Mercury Acalypha rhomboidea Raf. FACU Common Three‐Seed‐Mercury Acalypha virginica L. FACU Virginia Three‐Seed‐Mercury Acer negundo L. FAC Ash‐Leaf Maple Acer nigrum Michx. f. FACU Black Maple Acer pensylvanicum L. FACU Striped Maple Acer platanoides L. UPL Norway Maple Acer rubrum L. FAC Red Maple Acer saccharinum L. FACW Silver Maple Acer saccharum Marsh. FACU Sugar Maple Acer spicatum Lam. -
Violaceae – Violet Family
VIOLACEAE – VIOLET FAMILY Plant: small herbs, sometimes woody plants or vines in tropics Stem: rhizomes or stolons may be present, stems present or not Root: Leaves: simple, entire or toothed or lobed, alternate, rarely opposite or whorled; with stipules Flowers: mostly perfect, strongly irregular (zygomorphic); 5 sepals, persistent; 5 petals, in violets the lowest is usually wider, heavily veined, and extends back into a spur (or not), lateral petals often bearded; 5 stamens loosely united; ovary superior, 2-5 carpels, 1 style though often modified Fruit: berry or capsule or rarely a nut, fleshy or not Other: mostly tropical, our species mostly violets; Dicotyledons Group Genera: 21+ genera; locally Hybanthus (green violet), Viola (violet) WARNING – family descriptions are only a layman’s guide and should not be used as definitive Flower Morphology in the Genus Viola - examples Violaceae (Violet Family) 5-petaled flower, usually with a spur (or sac) as part of the lower petal in the Viola genus; The green violet (Cubelium genus) lacks a spur, flowers from nodes on stem Violet ID (Key) often starts with asking if the plant is Caulescent (Stemmed) – flower on Field Pansy [Johnny-Jump-Up] Viola bicolor Pursh. a leafy stem or Acaulescent (Stemless) – Birdfoot Violet flower on Scape, a stem without leaves Viola pedata L. (usually basal leaves only) Genus Cubelium Genus Viola Long-Spurred Violet Viola rostrata Pursh Sweet White Violet Viola blanda Willd. Eastern Green Violet Common [Wolly] Blue Violet Hybanthus concolor Viola Sororia Willd. (T.F. Forst.) Spreng. [Downy] Yellow Violet Striped [Cream] White Violet Viola Pubescens Ait. Viola striata Ait.