Evaluating 87Sr/86Sr Isotope Ratios and Sr Mass Fractions in Otoliths Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Entwurf Des Maßnahmenprogramms Für Den Bayerischen Anteil Am Flussgebiet Donau
Entwurf des Maßnahmenprogramms für den bayerischen Anteil am Flussgebiet Donau Bewirtschaftungszeitraum 2016–2021 Dokument zur Information und Anhörung der Öffentlichkeit Die Europäische Wasserrahmenrichtlinie und ihre Umsetzung in Bayern 1 Einführung Die Europäische Wasserrahmenrichtlinie verpflichtet die Mitgliedstaaten, für jede Flussgebietseinheit oder für den in ihr Hoheitsgebiet fallenden Teil einer internationalen Flussgebietseinheit ein Maßnahmenprogramm festzulegen, um die Ziele des Art. 4 WRRL zu verwirklichen. Explizit sind für Wasserkörper, die laut Risikoanalyse die Umweltziele gemäß WRRL bis 2021 voraussichtlich nicht erreichen, geeignete Maßnahmen vorzusehen bzw. die Inanspruchnahme von Ausnahmen nach Artikel 4 (4)/(5) WRRL zu prüfen. Der sachliche Inhalt des Maßnahmenprogramms sowie zugehörige Fristen werden durch Art. 11 WRRL bzw. § 82 Abs. 2 bis 6 WHG sowie § 84 WHG festgelegt. Der Begriff Maßnahme ist in der WRRL weit gefasst und umfasst nicht nur technische Maßnahmen, sondern auch rechtliche, administrative, ökonomische, kooperative, kommunikationsbezogene und sonstige Instrumente, die der Umsetzung der Richtlinie dienen. Grundsätzlich enthält das Maßnahmenprogramm folgende Arten von Maßnahmen (vgl. Art. 11 Abs. 3–5 WRRL): • Grundlegende Maßnahmen sind zu erfüllende Mindestanforderungen an den Gewässerschutz, die sich aus der Umsetzung bestehender gemeinschaftlicher, nationaler oder landesspezifischer Gesetzgebung – unabhängig von der WRRL – ableiten; • Ergänzende Maßnahmen sind Maßnahmen, die zusätzlich zu den grundlegenden -
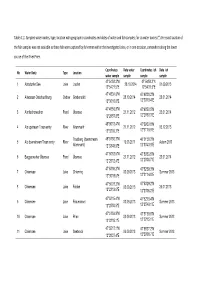
Sampled Water Bodies, Type, Location with Geographic
Table A.1: Sampled water bodies, type, location with geographic coordinates and dates of water and fish samples; for six water bodies (*) the exact location of the fish samples was not available as these fish were captured by fishermen within the investigated lakes, or in one occasion, somewhere along the lower course of the River Prien. Coordinates Date water Coordinates fish Date fish No Water Body Type Location water sample sample sample sample 47°54'52.3"N 47°54'52.3"N 1 Abtsdorfer See Lake Laufen 28.10.2014 01.09.2013 12°54'21.5"E 12°54'21.5"E 47°48'34.6"N 47°48'30.2"N 2 Altwasser Osterbuchberg Oxbow Grabenstätt 28.10.2014 23.01.2014 12°30'10.6"E 12°30'19.4"E 47°48'56.8"N 47°48'53.5"N 3 Almfischerweiher Pond Übersee 21.11.2012 23.01.2014 12°29'57.8"E 12°29'53.3"E 48°00'13.4"N 47°59'53.8"N 4 Alz upstream Traun entry River Altenmarkt 21.11.2012 05.12.2013 12°32'00.3"E 12°31'19.9"E Trostberg (downstream 48°01'50.2"N 48°01'50.2"N 5 Alz downstream Traun entry River 10.03.2011 Autum 2013 Altenmarkt) 12°33'43.9"E 12°33'43.9"E 47°50'52.5"N 47°50'53.5"N 6 Baggerweiher Übersee Pond Übersee 21.11.2012 23.01.2014 12°29'12.4"E 12°29'08.7"E 47°53'09.2"N 47°52'59.3"N 7 Chiemsee Lake Chieming 03.09.2013 Summer 2013 12°30'19.5"E 12°31'14.8"E 47°50'28.3"N 47°50'29.2"N 8 Chiemsee Lake Felden 03.09.2013 26.07.2013 12°23'12.6"E 12°23'06.2"E 47°52'15.4"N 47°52'10.4"N 9 Chiemsee Lake Fraueninsel 03.09.2013 Summer 2013 12°25'50.5"E 12°25'49.1"E 47°51'59.8"N 47°51'59.8"N 10 Chiemsee Lake Prien 03.09.2013 Summer 2013 12°22'15.1"E 12°22'15.1"E 47°55'17.1"N -

Landkreis Traunstein 5 3711 W I E 7 1 138 L G Verkehrsmengenkarte 2005 B
6 Howaschen 3 Vordorf Bergham Ü 1 Ober- 77419501 Tüßling 9534 15312 Hasel- 77 M Heiligenstatt 13350 2473 bach 245 Polling flossing 4527Monham 898 Moosen 2171 77409818 77409504 77419507 12 1862 77429442 206 Ebing AÖ S MÜ t B Wolfgrub 130 11 2 Haiming 1 24 Föhren- Unter- 2 3082 11954 08 78429118 AÖ 1787 0 77399819 2 Graming 77429432 4308 9 Unter- Piesing 0 0 winkel 5 77419811 schlottham 2 2 86 410 t 5 Wallner 9557 Riedbach 77409815 42 3 193 3176 Ü S 0 10498 2 Ober- M a.d.Osterwies 1 t 2129 M S M 77409506 Ö 77429436 1082 Ü 2599 Aigner Ü 241 Litzl- 4 A 870 Kemerting 3 1 7 2 WALDKRAI- Neue kirchen S 74 5 10522 Grünbach Ö 10363 t 164 A Emmerting M 2 Ü 0 Bergham 3 Wiesengrund 9 2 7 2 Heimat 538 5 1 550 Kastl Neuhofen 1 BURG 3 Frauendorf Klugham Waltenberg A 7 B Pürten Ö Reicherts- Thann Klugham 0 78429180 St 2 Guttenburg Bergham 6 1 Ober- 2 77409501 Forster t Seng St 2 M 2092 Kiefering S Hohenwart Öd 12936 heim Waldwinkel 35 Ü 18 St S 2 Weinthal t Aschau 10231 2 M Sankt Mörmoosen Schmid i.Lehen 7843 1 0 Ü 7842 8 1370 1 Erasmus Alten- M o o s 8 489 7841 7840 a.Inn 78409504 Ensdorf AÖ 2 2 Niederndorf buch 4 78409801 S 9 78419184 78409803 9 A S t t 21 7008 Fisslkling 2 08 2 Ö 78429171 78409800 3 B 2 5 1 4369 8139 10 B 7281 5 78419500 B 208 78429437 2 5590 0 22086 2 102 697 35 420 2587 Putz Lengthal 2 Maximilian t 9 2 12285 1619 S 1 270 209 Bergham Ü 78409802 St Gasteig 78429400 M Schützenau 128 6 Pirach 78409507 699 78419800 Ö 4 Reit 1 6 3960 A S 5 t 2 Oberdorf 107 t 23 3115 Kraiburg Ü Endfelln S 4898 MÜ 1 1223 159 4 M 215 Mehring 78429202 Hausing -

12. August 2009 ILG Fonds Nr. 35 Prospekt
sicher beteiligt 35Immobilien-Fonds Nr. Monatliche Auszahlung sicher beteiligt Immobilien-Fonds Nr. 35 Pflegeheim Traunstein Oberbayern Inhalt Seite Erklärung des Prospektherausgebers.................................................................................................................3 Der geschlossene Immobilienfonds als Kapitalanlage......................................................................................4 Gesellschaftliches Engagement und ertragsstarke Investition ........................................................................5 ILG-Immobilienfonds von 1980 bis 2009...........................................................................................................6 Das Angebot im Überblick..................................................................................................................................8 Beteiligungsstruktur.........................................................................................................................................12 Die Risiken.........................................................................................................................................................14 Die ILG als Fondsinitiator..................................................................................................................................18 ILG-Leistungsdaten zum 31.12.2007 ...............................................................................................................20 Investitionen in Pflegeimmobilien – ein Zukunftsmarkt................................................................................22 -

09-761 Eindrapport Forellen
A risk analysis of exotic trout in the Netherlands D. M. Soes P.-B. Broeckx Consultants for environment & ecology A risk analysis of exotic trout in the Netherlands D.M. Soes P.-B. Broeckx Commissioned by: Food and Consumer Product Safety Authority 9th of September 2010 Report nr 10-144 Status: Final report Report nr.: 10-144 Date of publication: 9th of September 2010 Title: A risk analysis of exotic trout in the Netherlands Author: Ir. D.M. Soes Ir. P.-B. Broeckx Number of pages without appendices: 96 Project nr: 09-761 Project manager: Ir. D.M. Soes Name & address client: Food and Consumer Product Safety Authority, Invasive Alien Species Team, P.O. Box 9102, 6700 HC, Wageningen Reference client: TRCPD/2009/3834 Signed for publication: General director Bureau Waardenburg bv drs. J.L. Spier Initials: Bureau Waardenburg bv is not liable for any resulting damage, nor for damage which results from applying results of work or other data obtained from Bureau Waardenburg bv; client indemnifies Bureau Waardenburg bv against third-party liability in relation to these applications. © Bureau Waardenburg bv / Food and Consumer Product Safety Authority This report is produced at the request of the client mentioned above and is his property. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, transmitted and/or publicized in any form or by any means, electronic, electrical, chemical, mechanical, optical, photocopying, recording or otherwise, without prior written permission of the client mentioned above and Bureau Waardenburg bv, nor may it without such a permission be used for any other purpose than for which it has been produced. -

Rediscovery of a Presumed Extinct
The Scientific Naturalist Ecology, 0(0), 2020, e03065 © 2020 by the Ecological Society of America they were therefore declared extinct according to IUCN criteria (Freyhof and Kottelat 2008a, b). Today, C. gut- turosus is considered a prime example for speciation reversal, whereby a change in environmental conditions, which are necessary to maintain reproductive isolation Rediscovery of a presumed extinct among evolutionarily young species, causes species to go species, Salvelinus profundus, after extinct by collapsing into a hybrid swarm (Vonlanthen et al. 2012; D. Frei, unpublished data). The simultaneous re-oligotrophication disappearance of the ecologically similar S. profundus led to the conclusion that it shared the fate of C. guttur- 1,2,3 1,2 CARMELA J. D OENZ AND OLE SEEHAUSEN osus (Vonlanthen et al. 2012). Manuscript received 6 December 2019; revised 29 January However, in 2012, a colleague, Jasminca Behrmann- 2020; accepted 25 February 2020. Corresponding Editor: John Godel, informed us about a small, pale, summer- Pastor. spawning charr that she and others had caught. On 1 Department of Fish Ecology & Evolution, Centre for several previous occasions, small individuals of S. um- Ecology, Evolution and Biogeochemistry, EAWAG, bla had wrongly been identified as S. profundus (Kotte- Kastanienbaum, 6047 Switzerland. lat and Freyhof 2007), so in this case, we were hopeful 2Department of Aquatic Ecology & Evolution, Institute of that it could be the presumably extinct species, since it Ecology and Evolution, University of Bern, Bern, 3012 Switzerland. showed several of its traits. In 2010, a large project 3E-mail: [email protected] (Projet Lac) started to assess the fish community for each of the large pre-alpine lakes in and around Citation: Doenz, C. -

Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe
Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Lara Ibrahim, M.Sc. aus Mazeraat Assaf, Libanon Berichter: Universitätsprofessor Dr. Andreas Schäffer Prof. Dr. Christoph Schäfers Tag der mündlichen Prüfung: 30. Juli 2015 Diese Dissertation ist auf den Internetseiten der Universitätsbibliothek online verfügbar Erklärung Ich versichere, dass ich diese Doktorarbeit selbständig und nur unter Verwendung der angegebenen Hilfsmittel angefertigt habe. Weiterhin versichere ich, die aus benutzten Quellen wörtlich oder inhaltlich entnommenen Stellen als solche kenntlich gemacht zu haben. Lara Ibrahim Aachen, am 18 März 2015 Zusammenfassung Die Zulassung von Pflanzenschutzmitteln in der Europäischen Gemeinschaft verlangt unter anderem eine Abschätzung des Risikos für Organismen in der Umwelt, die nicht Ziel der Anwendung sind. Unvertretbare Auswirkungen auf den Naturhalt sollen vermieden werden. Die ökologische Risikoanalyse stellt die dafür benötigten Informationen durch eine Abschätzung der Exposition der Organismen und der sich daraus ergebenden Effekte bereit. Die Effektabschätzung beruht dabei hauptsächlich auf standardisierten ökotoxikologischen Tests im Labor mit wenigen, oft nicht einheimischen Stellvertreterarten. In diesen Tests werden z. B. Effekte auf das Überleben, das Wachstum und/oder die Reproduktion von Fischen bei verschiedenen Konzentrationen der Testsubstanz gemessen und Endpunkte wie die LC50 (Lethal Concentrations for 50%) oder eine NOEC (No Observed Effect Concentration, z. B. für Wachstum oder Reproduktionsparameter) abgeleitet. Für Fische und Wirbeltiere im Allgemeinen beziehen sich die spezifischen Schutzziele auf das Überleben von Individuen und die Abundanz und Biomasse von Populationen. -

Vierte Berichtigung Der Verzeichnisse Der Gewässer Zweiter Ordnung Und Der Wildbäche
Bayerisches Ministerialblatt BayMBl. 2020 Nr. 692 2. Dezember 2020 Vierte Berichtigung der Verzeichnisse der Gewässer zweiter Ordnung und der Wildbäche Bekanntmachung des Bayerischen Staatsministeriums für Umwelt und Verbraucherschutz vom 4. November 2020, Az. 52g-U4502-2010/3-177 1. Verfügung 1Nach Nr. 5 der Bekanntmachung des Bayerischen Staatsministeriums für Umwelt und Verbraucherschutz über die Verzeichnisse der Gewässer zweiter Ordnung und der Wildbäche vom 12. Februar 2016 (AllMBl. S. 150) werden die Gewässerverzeichnisse von Amts wegen überprüft und berichtigt. 2Hiermit werden die Verzeichnisse der Gewässer zweiter Ordnung und der Wildbäche (Anlagen 1 bis 3) berichtigt und neu erlassen. 2. Begründung 1Im Zuge des Abgleichs des Internet-Kartendiensts mit den Inhalten der Anlagen 1 bis 3 der Allgemeinverfügung vom 12. Dezember 2019 hat sich ein Korrekturbedarf ergeben. 2Von den Wasserwirtschaftsämtern wurden zu den Gewässern zweiter Ordnung die Korrektur eines Gewässernamens sowie einer Beschreibung des Endpunktes eines Gewässers gemeldet. 3Zu den Wildbächen wurden noch fehlende, neue und geänderte ausgebaute Wildbachstrecken sowie Änderungen der Ausbaulängen gemeldet. 4Zwei Wildbacheinzugsgebiete wurden aus dem Wildbachverzeichnis entfernt, da es sich um Gewässer zweiter Ordnung handelt, zwei Einzugsgebiete wurden an ihrem Endpunkt verändert. 5In der Anlage 1 (Verzeichnis der Gewässer zweiter Ordnung) gab es somit eine Namenskorrektur und eine Änderung der Beschreibung des Endpunkts. 6In der Anlage 2 (Wildbäche) erfolgte die Löschung von zwei Wildbacheinzugsgebieten, die Korrektur eines Einzugsgebietsnamens sowie die Änderung der Beschreibung des Endpunktes von zwei Einzugsgebieten. 7In die Anlage 3 (Ausgebaute Wildbachstrecken) wurden neben den von den Wasserwirtschaftsämtern gemeldeten drei Änderungen der ausgebauten Wildbachstrecken auch insgesamt zwölf neue ausgebaute Wildbachstrecken mit aufgenommen. 8Zwei Ausbaustrecken wurden gelöscht. -

Anlage 2: Wildbäche
Anlage 2 Wildbäche lfd. Kenn-Nr. Einzugsgebiet Anfangspunkt Endpunkt Bemerkungen Nr. 1 Regierungsbezirk Oberbayern (41) 2 Wildbäche im Amtsbereich des WWA München (412) 3 412001 Klosterschluchtgraben Kreuzung mit der Bundesstraße 11 in Unteres Ende des Schluchtlaufs ca. 200 m Ebenhausen, Gde. Schäftlarn, Lkr. westlich der Staatsstraße 207, Gde. München Schäftlarn, Lkr. München 4 Wildbäche im Amtsbereich des WWA Rosenheim (413) 5 413021 Achen (Thalkirchner Ache) Ursprung beim Stöttener Filz, Gde. Mündung in den Simssee, Markt Bad Frasdorf, Lkr. Rosenheim Endorf, Lkr. Rosenheim 6 413093 Almgraben Ursprung zwischen kleinem Tegernseer Mündung in den Tegernsee, Stadt Berg und Riederstein, Stadt Tegernsee, Tegernsee, Lkr. Miesbach Lkr. Miesbach 7 413087 Alpbach (MB) Ursprung zwischen Ostiner Berg, Mündung in den Tegernsee, Stadt Baumgartenschneid und kleinem Tegernsee, Lkr. Miesbach Tegernseer Berg, Stadt Tegernsee, Lkr. Miesbach 8 413080 Altdorfer Mühlbach Straßendurchlass unterhalb Gern, Gde. Mündung in den Nasenbach, Markt Gars Soyen, Lkr. Rosenheim a.Inn, Lkr. Mühldorf a.Inn 9 413048 Ameranger Dorfbach Ursprünge nördlich und südwestlich von Brücke Gemeindestraße Amerang - Taiding, Gde. Amerang, Lkr. Rosenheim Kammer, Gde. Amerang, Lkr. Rosenheim 10 413024 Angerbach (RO) Ursprung östlich von Haring, Gde. Mündung in den Simssee, Gde. Riedering, Riedering, Lkr. Rosenheim Lkr. Rosenheim 11 413025 Antworter Berg: Gräben am Ursprung am Antworter Berg, Markt Bad Mündung in die Antworter Ache bzw. Nordwesthang Endorf, Lkr. Rosenheim Einlauf in die Rohrleitung südlich von Antwort, Markt Bad Endorf, Lkr. Rosenheim 12 413045 Aubach (RO) Ursprung auf der Niklasreuther Höhe, Gde. Ehemalige Bahnbrücke unterhalb von Au, Ausgenommen Zufluss aus Irschenberg, Lkr. Miesbach Gde. Bad Feilnbach, Lkr. Rosenheim Brettschleipfen 13 413006 Auerbach Ursprung östlich des Tagweidkopfes, Gde. -

Managing Upper Lake Constance Fishery in a Multi- Sector Policy Landscape: Beneficiary and Victim of a Century of Anthropogenic Trophic Change
Managing Upper Lake Constance Fishery in a Multi- Sector Policy Landscape: Beneficiary and Victim of a Century of Anthropogenic Trophic Change Jan Baer1, Reiner Eckmann2, Roland Rösch1, Robert Arlinghaus3 & Alexander Brinker1 1Fisheries Research Station, Langenargen, Germany, [email protected] 2University of Konstanz, Germany 3Faculty of Life Sciences, Humboldt-Universität zu Berlin & Department of Biology and Ecology of Fishes, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Germany Abstract Upper Lake Constance (ULC) is a large pre-alpine lake situated between Austria, Germany and Switzerland (9°18'E, 47°39'N). Along with the smaller, conjoined expanse of Lower Lake Constance, it forms the third largest lake in Europe. Its waters underwent pronounced eutrophication during the 20th century. Commercial fisheries benefitted strongly from the increased productivity during an initial mesotrophic phase, but these advantages were effectively neutralized when eutrophication became severe. By the turn of the 21st century, internationally coordinated measures to reduce nutrient input to the lake had returned ULC to its historic reference state as an oligotrophic ecosystem. However, the remarkable success of the nutrient management program has been to the detriment of commercial fishers. Yields of most commercially important fish species have decreased, along with lake productivity. As a consequence, the high market demand for local fish products is nowadays met mainly by imports, the ecological footprint of which offsets the local benefits of environmental restoration. Responsibility for fisheries and environmental aspects of ULC managing is shared by the national and federal state administrations and in all cases, tourism, drinking water and environmental interests now take priority over fisheries. -

Seeon-Seebruck, Truchtlaching
Seeon-Seebruck, TruchTlaching Seebruck* – Die Sportliche • Großes Strandbad mit 30.000 qm liegewie- Wer in Seebruck am ufer des chiemsees steht se – ideal auch für kinder geeignet und nach Süden schaut, dem öffnet sich die gesamte Weite des „bayerischen Meeres“, an Seeon** – Die Kulturelle klaren Tagen ein überwältigender blick auf es sind vierzehn Seen, die das naturschutzge- die alpen von den berchtesgadener alpen bis biet „Seeoner Seen“ bilden – reste eiszeit- hin zum Wendelstein. licher gewässer – nördlich des chiemsees Für einen aktiven und interessanten urlaub gelegen. Die Schönheit und den reiz dieser bieten wir unseren gästen: landschaft schätzten bereits vor über 1000 • Römermuseum „bedaium“ und ausgangs- Jahren die benediktiner und so gründeten sie punkt des 27 km langen „archäologischen 994 das kloster Seeon. bis zur Säkularisation Rundweges“ durch die 3 orte 1803 war das kloster ein ort der gelehrsam- • „heinrich-kirchner galerie“ und beginn keit und kultur. So war auch W.a. Mozart des neuen „heinrich kirchner Skulpturen- häufig zu gast und komponierte hier einige weges“ seiner Werke. • Paradies für den Wassersport – Segeln, Seeon bietet seinen gästen: Surfen und Schwimmen • Klosterkirche St. lambert mit kreuzgang *staatl. anerkannter luftkurort • Segelhafen mit 500 Wasser- und Trocken- • Kirche St. Walburgis mit dem russisch- **staatl. anerkannter erholungsort liegeplätzen (auch gastliegeplätze) orthodoxen Friedhof 56 Seeon-Seebruck und Truchtlaching Seeon – umringt von sieben Seen, Seebruck – badeort seit der römerzeit, Truchtlaching – im landschaftsschutzgebiet Peterskirchen Engelsberg Wiesmühl des oberen alztales gelegen – herzlich willkommen in ihrem Tacherting urlaubsparadies! Emertsham Harpfing Kienberg Trostberg Frabertsham • Eine Vielzahl von konzerten, ausstel- gehoben. Truchtlaching bietet seinen gästen: Palling Altenmarkt a. d. Alz Obing i Stein a. -

An Annotated Bibliography of Interspecific Hybridization
FAO Fisheries Circular No.133 FIRI/C133 (Distribution restricted) AN ANNOTATED BIBLIOGRAPHY OF INTERSPECIFIC HYBRIDIZATION OF SALMONIDAE Compiled by James R. Dangel College of Fisheries University of Washington FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, September 1973 PREPARATION OF THIS DOCUMENT This bibliography is an attempt by the author to include all known literature, pub- lished and unpublished, on hybridization in Salmonidae. The whitefishes and graylings are considered by the author as separate families and are not included. The author would appreciate being informed of any references to salmonoid hybrids known to the reader that are not included in this bibliography, as well as corrections or additions to the annotations, so that tuey may be included in future revisions or addenda. Articles not obtained for review are included but not annotated unless referred to in other sources. When abstracts or summaries pertaining to hybridization were included in papers, they have been transcribed in quotation marks verbatim, as are certain passages of text when applicable. Unless otherwise cited, the abstracts were written by the author. WI/E2219 FAO Fisheries Circular (FAO Fish.Circ.) A vehicle for distribution of short or ephemeral notes, lists, etc., including provisional versions of documents to be issued later in other series. 1 Ackermann, K. (1898) 001 Alm, G. (1959) 006 Abh.Ber.Ver.Naturkd.Kassel, 43:4-11 Rep.Inst.Freshwat.Res.Drottningholm, Thierbastarde. Zusammenstellung der T40):5-145 bisherigen Beobachtungen Uber Bastardirung Connection between maturity, size and im Thierreiche nebst Litteraturnachweisen. age in fishes 2. Theil: Die Wirbelthiere (Fische) Salmo salar and S.