09-761 Eindrapport Forellen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Solution for Universal Classification of Species Based on Genomic
Hindawi Publishing Corporation International Journal of Plant Genomics Volume 2007, Article ID 27894, 8 pages doi:10.1155/2007/27894 Research Article A Solution for Universal Classification of Species Based on Genomic DNA Mariko Kouduka,1 Daisuke Sato,1 Manabu Komori,1 Motohiro Kikuchi,2 Kiyoshi Miyamoto,3 Akinori Kosaku,3 Mohammed Naimuddin,1, 4 Atsushi Matsuoka,5 and Koichi Nishigaki1, 6 1 Department of Functional Materials Science, Saitama University, Saitama, Japan 2 Chitose Salmon Aquarium Chitose, Youth Educational Foundation, Chitose, Hokkaido, Japan 3 Institute of Medical Science, Dokkyo Medical University, Tochigi, Japan 4 Biol. Res. and Functions, National Inst. AIST, Tsukuba, Ibaraki, Japan 5 Department of Geology, Niigata University, Niigata, Japan 6 Rational Evolutionary Design of Advanced Biomolecules, Saitama Small Enterprise Promotion Corporation, SKIP City, Saitama, Japan Received 22 July 2006; Revised 8 October 2006; Accepted 8 October 2006 Recommended by Cheng-Cang Wu Traditionally, organisms have been classified on the basis of their phenotype. Recently, genotype-based classification has become possible through the development of sequencing technology. However, it is still difficult to apply sequencing approaches to the analysis of a large number of species due to the cost and labor. In most biological fields, the analysis of complex systems compris- ing various species has become an important theme, demanding an effective method for handling a vast number of species. In this paper, we have demonstrated, using plants, fish, and insects, that genome profiling, a compact technology for genome analysis, can classify organisms universally. Surprisingly, in all three of the domains of organisms tested, the phylogenetic trees generated from the phenotype topologically matched completely those generated from the genotype. -

Cottus Poecilopus Heckel, 1836, in the River Javorin- Ka, the Tatra
Oecologia Montana 2018, Cottus poecilopus Heckel, 1836, in the river Javorin- 27, 21-26 ka, the Tatra mountains, Slovakia M. JANIGA, Jr. In Tatranská Javorina under Muráň mountain, a small fish nursery was built by Christian Kraft von Institute of High Mountain Biology University of Hohenlohe around 1930. The most comprehensive Žilina, Tatranská Javorina 7, SK-059 56, Slovakia; studies on fish from the Tatra mountains were writ- e-mail:: [email protected] ten by professor Václav Dyk (1957; 1961), Dyk and Dyková (1964a,b; 1965), who studied altitudinal distribution of fish, describing the highest points where fish were found. His studies on fish were likely the most complex studies of their kind during that period. Along with his wife Sylvia, who illus- Abstract. This study focuses on the Cottus poe- trated his studies, they published the first realistic cilopus from the river Javorinka in the north-east studies on fish from the Tatra mountains including High Tatra mountains, Slovakia. The movement the river Javorinka (Dyk and Dyková 1964a). Feri- and residence of 75 Alpine bullhead in the river anc (1948) published the first Slovakian nomenclature were monitored and carefully recorded using GPS of fish in 1948. Eugen K. Balon (1964; 1966) was the coordinates. A map representing their location in next famous ichthyologist who became a recognised the river was generated. This data was collected in expert in the fish fauna of the streams of the Tatra the spring and summer of 2016 and in the autumn mountains, the river Poprad, and various high moun- of 2017. Body length and body weight of 67 Alpine tain lakes. -

Use of Stream Mouth Habitats by Cottus Perifretum and Leuciscus Cephalus Along the River Meuse (The Netherlands)
Folia Zool. – 59(1): 44 –50 (2010) Use of stream mouth habitats by Cottus perifretum and Leuciscus cephalus along the River Meuse (the Netherlands) Bart J.A. Pollux1* and Anikó Kőrösi2 1Department of Biology, University of California Riverside, 900 University Avenue, 2930 Life Sciences Psychology, Riverside, CA 92521 USA; e-mail: [email protected], [email protected] 2Department of Cellular Animal Physiology, Radboud University Nijmegen, Toernooiveld 1, 6525 ED Nijmegen, The Netherlands received 22 July 2008; Accepted 31 August 2009 Abstract. size-frequency data were collected for two rheophilic fish species, Cottus perifretum and Leuciscus cephalus, at the confluences of 18 lowland tributaries along the regulated river Meuse (the Netherlands) between May 2004 and April 2005. Cottus perifretum is a resident species, using these stream mouth habitats throughout its entire life: i.e. as a spawning, nursery and adult habitat. Leuciscus cephalus is a transient species that uses these stream mouth habitats only as a temporary 0+ juvenile habitat during fall and early winter. This study suggests that the stream mouth habitats along the river Meuse fulfil different ecological functions forC. perifretum and L. cephalus. Key words: larvae, juveniles, nursery, resident, spawning, transient Introduction Velde et al. 1990, Admiraal et al. 1993, Van den Brink et al. 1996, raat 2001). over the last two centuries, extensive changes it has been argued that off-channel water to the geomorphology of large lowland rivers bodies connected to the main channel, such as in the Netherlands have resulted in a severe floodplain lakes (Grift et al. 2003), gravel pit loss of habitat heterogeneity (Admiraal et lakes (Neumann et al. -

Evaluating 87Sr/86Sr Isotope Ratios and Sr Mass Fractions in Otoliths Of
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.23.453494; this version posted July 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Evaluating 87Sr/86Sr isotope ratios and Sr mass fractions in otoliths of different European freshwater 2 fish species as fishery management tool in an Alpine foreland with limited geological variability 3 Andreas Zitek1,2*, Johannes Oehm3, Michael Schober1, Anastassiya Tchaikovsky1, Johanna Irrgeher5, 4 Anika Retzmann5, Bettina Thalinger4, Michael Traugott3, Thomas Prohaska5 5 1University of Natural Resources and Life Sciences, Vienna, Department of Chemistry, Institute of 6 Analytical Chemistry, Muthgasse 18, 1190 Wien, Austria 7 2FFoQSI GmbH ‐ Austrian Competence Centre for Feed and Food Quality, Safety & Innovation, 8 Technopark 1D, 3430 Tulln, Austria 9 3University of Innsbruck, Department of Zoology, Technikerstraße 25, 6020 Innsbruck, Austria 10 4University of Guelph, 50 Stone Road East, Guelph, N1G2W1, Canada 11 5Department of General, Analytical and Physical Chemistry, Chair of General and Analytical Chemistry, 12 Montanuniversität Leoben, Franz Josef‐Straße 18, 8700 Leoben, Austria 13 14 *Corresponding author: [email protected] 15 16 Highlights 17 Otolith microchemistry applied in in area with limited geological variability 18 Fish transferred, stocked or migrated were identified 19 Regressions between Sr/Ca ratios in water predict Sr mass fractions in otoliths 20 Species specific Sr discrimination from water into otoliths 21 European freshwater fish species assigned to habitat clusters of origin 22 Keywords 23 Strontium isotopes, Sr elemental fingerprint, otolith microchemistry, freshwater fish species, fishery 24 management. -
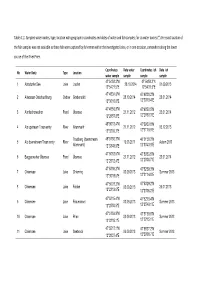
Sampled Water Bodies, Type, Location with Geographic
Table A.1: Sampled water bodies, type, location with geographic coordinates and dates of water and fish samples; for six water bodies (*) the exact location of the fish samples was not available as these fish were captured by fishermen within the investigated lakes, or in one occasion, somewhere along the lower course of the River Prien. Coordinates Date water Coordinates fish Date fish No Water Body Type Location water sample sample sample sample 47°54'52.3"N 47°54'52.3"N 1 Abtsdorfer See Lake Laufen 28.10.2014 01.09.2013 12°54'21.5"E 12°54'21.5"E 47°48'34.6"N 47°48'30.2"N 2 Altwasser Osterbuchberg Oxbow Grabenstätt 28.10.2014 23.01.2014 12°30'10.6"E 12°30'19.4"E 47°48'56.8"N 47°48'53.5"N 3 Almfischerweiher Pond Übersee 21.11.2012 23.01.2014 12°29'57.8"E 12°29'53.3"E 48°00'13.4"N 47°59'53.8"N 4 Alz upstream Traun entry River Altenmarkt 21.11.2012 05.12.2013 12°32'00.3"E 12°31'19.9"E Trostberg (downstream 48°01'50.2"N 48°01'50.2"N 5 Alz downstream Traun entry River 10.03.2011 Autum 2013 Altenmarkt) 12°33'43.9"E 12°33'43.9"E 47°50'52.5"N 47°50'53.5"N 6 Baggerweiher Übersee Pond Übersee 21.11.2012 23.01.2014 12°29'12.4"E 12°29'08.7"E 47°53'09.2"N 47°52'59.3"N 7 Chiemsee Lake Chieming 03.09.2013 Summer 2013 12°30'19.5"E 12°31'14.8"E 47°50'28.3"N 47°50'29.2"N 8 Chiemsee Lake Felden 03.09.2013 26.07.2013 12°23'12.6"E 12°23'06.2"E 47°52'15.4"N 47°52'10.4"N 9 Chiemsee Lake Fraueninsel 03.09.2013 Summer 2013 12°25'50.5"E 12°25'49.1"E 47°51'59.8"N 47°51'59.8"N 10 Chiemsee Lake Prien 03.09.2013 Summer 2013 12°22'15.1"E 12°22'15.1"E 47°55'17.1"N -

Error-Robust Nature of Genome Profiling Applied for Clustering of Species Demonstrated by Computer Simulation
World Academy of Science, Engineering and Technology International Journal of Bioengineering and Life Sciences Vol:1, No:5, 2007 Error-Robust Nature of Genome Profiling Applied for Clustering of Species Demonstrated by Computer Simulation Shamim Ahmed and Koichi Nishigaki factors [4], and the insufficiency in the number of experts [5]. Abstract—Genome profiling (GP), a genotype based technology, Recently, genotype-based approach has become possible which exploits random PCR and temperature gradient gel owing to the development of sequencing technology. electrophoresis, has been successful in identification/classification of organisms. In this technology, spiddos (Species identification dots) However, it is still difficult to apply sequencing approaches to and PaSS (Pattern similarity score) were employed for measuring the the analysis of a large number of species due to logistic closeness (or distance) between genomes. Based on the closeness reason. In most biological fields, the analysis of complex (PaSS), we can buildup phylogenetic trees of the organisms. We systems comprising various species has been an important noticed that the topology of the tree is rather robust against the experimental fluctuation conveyed by spiddos. This fact was theme, demanding an effective method for handling a vast confirmed quantitatively in this study by computer-simulation, number of species. A realistic solution to these problems has providing the limit of the reliability of this highly powerful been to characterize organisms according to the sequence of methodology. As a result, we could demonstrate the effectiveness of their small subunit ribosomal RNA (16S/18S rRNA), an the GP approach for identification/classification of organisms. approach that has been applied to various organisms, initiated Keywords—Fluctuation, Genome profiling (GP), Pattern by Woese and his collaborators [6]~[8]. -

Dean Oz/Μ: ;Z: Date
The evolutionary history of reproductive strategies in sculpins of the subfamily oligocottinae Item Type Thesis Authors Buser, Thaddaeus J. Download date 26/09/2021 18:39:58 Link to Item http://hdl.handle.net/11122/4549 THE EVOLUTIONARY HISTORY OF REPRODUCTIVE STRATEGIES IN SCULPINS OF THE SUBFAMILY OLIGOCOTTINAE By Thaddaeus J. Buser RECOMMENDED: Dr. Anne Beaudreau Dr. J. Andres Lopez Advisory Committee Chair Dr. Shannon Atkinson Fisheries Division Graduate Program Chair APPROVED: Dr. Michael Castellini ·. John Eichel erger Dean oZ/µ:_;z: Date THE EVOLUTIONARY HISTORY OF REPRODUCTIVE STRATEGIES IN SCULPINS OF THE SUBFAMILY OLIGOCOTTINAE A THESIS Presented to the Faculty of the University of Alaska Fairbanks in Partial Fulfillment of the Requirements for the Degree of Title Page MASTER OF SCIENCE By Thaddaeus J. Buser, B.Sc. Fairbanks, Alaska May 2014 v Abstract The sculpin subfamily Oligocottinae is a group of 17 nearshore species and is noteworthy for the fact that it contains both intertidal and subtidal species, copulating and non- copulating species, and many species with very broad geographic ranges. These factors, as well as the consistency with which the constituent genera have been grouped together historically, make the Oligocottinae an ideal group for the study of the evolution of a reproductive mode known as internal gamete association (IGA), which is unique to sculpins. I conducted a phylogenetic study of the oligocottine sculpins based on an extensive molecular dataset consisting of DNA sequences from eight genomic regions. From the variability present in those sequences, I inferred phylogenetic relationships using parsimony, maximum likelihood, and Bayesian inference. Results of these phylogenetic analyses show that some historical taxonomy and classifications require revision to align taxonomy with evolutionary relatedness. -

Ichthyofauna of the Kubo, Tochikura, and Ichinono
Biodiversity Data Journal 2: e1093 doi: 10.3897/BDJ.2.e1093 Taxonomic paper Ichthyofauna of the Kubo, Tochikura, and Ichinono river systems (Kitakami River drainage, northern Japan), with a comparison of predicted and surveyed species richness Yusuke Miyazaki†,‡, Masanori Nakae§†, Hiroshi Senou † Kanagawa Prefectural Museum of Natural History, Kanagawa, Japan ‡ The University of Tokyo, Tokyo, Japan § National Museum of Nature and Science, Ibaraki, Japan Corresponding author: Yusuke Miyazaki ([email protected]) Academic editor: Rupert Collins Received: 28 Mar 2014 | Accepted: 23 Oct 2014 | Published: 07 Nov 2014 Citation: Miyazaki Y, Nakae M, Senou H (2014) Ichthyofauna of the Kubo, Tochikura, and Ichinono river systems (Kitakami River drainage, northern Japan), with a comparison of predicted and surveyed species richness. Biodiversity Data Journal 2: e1093. doi: 10.3897/BDJ.2.e1093 Abstract The potential fish species pool of the Kubo, Tochikura, and Ichinono river systems (tributaries of the Iwai River, Kitakami River drainage), Iwate Prefecture, northern Japan, was compared with the observed ichthyofauna by using historical records and new field surveys. Based on the literature survey, the potential species pool comprised 24 species/ subspecies but only 20, including 7 non-native taxa, were recorded during the fieldwork. The absence during the survey of 11 species/subspecies from the potential species pool suggested either that sampling effort was insufficient, or that accurate determination of the potential species pool was hindered by lack of biogeographic data and ecological data related to the habitat use of the species. With respect to freshwater fish conservation in the area, Lethenteron reissneri, Carassius auratus buergeri, Pseudorasbora pumila, Tachysurus tokiensis, Oryzias latipes, and Cottus nozawae are regarded as priority species, and Cyprinus rubrofuscus, Pseudorasbora parva, and Micropterus salmoides as targets for removal. -

Gemeentelijk Rioleringsplan Eijsden-Margraten 2018-2022
Gemeentelijk Rioleringsplan Eijsden-Margraten 2018-2022 Stedelijk afvalwater, hemelwater en grondwater Ontwerp Sweco T +31 88 811 66 00 Sweco Nederland B.V. Karst Jan van Esch De Molen 48 www.sweco.nl Houten 3994 DB Houten Handelsregister 30129769 T +31 88 811 43 39 Postbus 4090 Statutair gevestigd te De Bilt M +31 6 22 46 63 61 3502 HB Utrecht Verantwoording Titel Gemeentelijk rioleringsplan Eijsden- Margraten 2018-2022 Subtitel Stedelijk afvalwater, hemelwater en grondwater Projectnummer 353184 Referentienummer Referentienummer Revisie O2 Datum 08-09-2017 Auteur(s) Ir. Karst Jan van Esch, dr.ir. Wouter van Riel E-mailadres [email protected] Gecontroleerd door Elwin Leusink MSc Paraaf gecontroleerd Goedgekeurd door Stephan Jansen Paraaf goedgekeurd nl_rapport.docx 20161201 2 (78 ) Inhoudsopgave 1 Een nieuw gemeentelijk rioleringsplan ............................................................... 5 1.1 Waarom is een gemeentelijk rioleringsplan belangrijk? .......................................... 5 1.2 Een gezamenlijk gemeentelijk rioleringsplan .......................................................... 5 1.3 Voor wie is het GRP? .............................................................................................. 6 1.4 Wat staat er in dit gemeentelijk rioleringsplan? ....................................................... 6 2 Even terug kijken ................................................................................................... 7 2.1 Wat hebben we bereikt? ......................................................................................... -

Armies and Ecosystems in Premodern Europe: the Meuse Region, 1250–1850
WCP ARMIES AND ECOSYSTEMS IN PREMODERN EUROPE THE MEUSE REGION, 1250–1850 Using the ecosystem concept as his starting point, the author examines the complex relationship between premodern armed forces and their environ- and Conflict in War ment at three levels: landscapes, living beings, and diseases. The study focuses Societies Premodern on Europe’s Meuse Region, well-known among historians of war as a battle- ground between France and Germany. By analyzing soldiers’ long-term inter- actions with nature, this book engages with current debates about the eco- ARMIES AND ECOSYSTEMS IN PREMODERN EUROPE IN PREMODERN logical impact of the military, and provides new impetus for contemporary armed forces to make greater effort to reduce their environmental footprint. “This is an impressive interdisciplinary study, contributing to environmental history, the history of war and historical geography. The book advances an original and intriguing argument that armed forces have had a vested interest in preserving the environments and habitats in which they operate, and have thus contributed to envi- ronmental conservation long before this became a popular cause of wider humanity. The work will provide a template for how this topic can be researched for other parts of the world or for other time periods.” Peter H. Wilson, Chichele Professor of the History of War, University of Oxford War and Confl ict in Premodern Societies is a pioneering series that moves away from strategies, battles, and chronicle histories in order to provide a home for work that places warfare in broader contexts, and contributes new insights ARMIES AND ECOSYSTEMS on everyday experiences of confl ict and violence. -

Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A
Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France A. Maire, P. Laffaille, J.F. Maire, L. Buisson To cite this version: A. Maire, P. Laffaille, J.F. Maire, L. Buisson. Identification of Priority Areas for the Conservation of Stream Fish Assemblages: Implications for River Management in France. River Research and Applications, Wiley, 2016, 33 (4), pp.524-537. 10.1002/rra.3107. hal-01426354 HAL Id: hal-01426354 https://hal.archives-ouvertes.fr/hal-01426354 Submitted on 2 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License IDENTIFICATION OF PRIORITY AREAS FOR THE CONSERVATION OF STREAM FISH ASSEMBLAGES: IMPLICATIONS FOR RIVER MANAGEMENT IN FRANCE A. MAIREa*,†, P. LAFFAILLEb,c, J.-F. MAIREd AND L. BUISSONb,e a Irstea; UR HYAX, Pôle Onema-Irstea Hydroécologie des plans d’eau; Centre d’Aix-en-Provence, Aix-en-Provence, France b CNRS; UMR 5245 EcoLab, (Laboratoire Ecologie Fonctionnelle et Environnement), Toulouse, France c Université de Toulouse, INP, UPS; EcoLab; ENSAT, Castanet Tolosan, France d ONERA, The French Aerospace Lab Composites Department, Châtillon, France e Université de Toulouse, INP, UPS; EcoLab, Toulouse, France ABSTRACT Financial and human resources allocated to biodiversity conservation are often limited, making it impossible to protect all natural places, and priority areas for protection must be identified. -

Rediscovery of a Presumed Extinct
The Scientific Naturalist Ecology, 0(0), 2020, e03065 © 2020 by the Ecological Society of America they were therefore declared extinct according to IUCN criteria (Freyhof and Kottelat 2008a, b). Today, C. gut- turosus is considered a prime example for speciation reversal, whereby a change in environmental conditions, which are necessary to maintain reproductive isolation Rediscovery of a presumed extinct among evolutionarily young species, causes species to go species, Salvelinus profundus, after extinct by collapsing into a hybrid swarm (Vonlanthen et al. 2012; D. Frei, unpublished data). The simultaneous re-oligotrophication disappearance of the ecologically similar S. profundus led to the conclusion that it shared the fate of C. guttur- 1,2,3 1,2 CARMELA J. D OENZ AND OLE SEEHAUSEN osus (Vonlanthen et al. 2012). Manuscript received 6 December 2019; revised 29 January However, in 2012, a colleague, Jasminca Behrmann- 2020; accepted 25 February 2020. Corresponding Editor: John Godel, informed us about a small, pale, summer- Pastor. spawning charr that she and others had caught. On 1 Department of Fish Ecology & Evolution, Centre for several previous occasions, small individuals of S. um- Ecology, Evolution and Biogeochemistry, EAWAG, bla had wrongly been identified as S. profundus (Kotte- Kastanienbaum, 6047 Switzerland. lat and Freyhof 2007), so in this case, we were hopeful 2Department of Aquatic Ecology & Evolution, Institute of that it could be the presumably extinct species, since it Ecology and Evolution, University of Bern, Bern, 3012 Switzerland. showed several of its traits. In 2010, a large project 3E-mail: [email protected] (Projet Lac) started to assess the fish community for each of the large pre-alpine lakes in and around Citation: Doenz, C.