1. Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Telephone Numbers
DISTRICT DISASTER MANAGEMENT AUTHORITY THANJAVUR IMPORTANT TELEPHONE NUMBERS DISTRICT EMERGENCY OPERATION CENTRE THANJAVUR DISTRICT YEAR-2018 2 INDEX S. No. Department Page No. 1 State Disaster Management Department, Chennai 1 2. Emergency Toll free Telephone Numbers 1 3. Indian Meteorological Research Centre 2 4. National Disaster Rescue Team, Arakonam 2 5. Aavin 2 6. Telephone Operator, District Collectorate 2 7. Office,ThanjavurRevenue Department 3 8. PWD ( Buildings and Maintenance) 5 9. Cooperative Department 5 10. Treasury Department 7 11. Police Department 10 12. Fire & Rescue Department 13 13. District Rural Development 14 14. Panchayat 17 15. Town Panchayat 18 16. Public Works Department 19 17. Highways Department 25 18. Agriculture Department 26 19. Animal Husbandry Department 28 20. Tamilnadu Civil Supplies Corporation 29 21. Education Department 29 22. Health and Medical Department 31 23. TNSTC 33 24. TNEB 34 25. Fisheries 35 26. Forest Department 38 27. TWAD 38 28. Horticulture 39 29. Statisticts 40 30. NGO’s 40 31. First Responders for Vulnerable Areas 44 1 Telephone Number Officer’s Details Office Telephone & Mobile District Disaster Management Agency - Thanjavur Flood Control Room 1077 04362- 230121 State Disaster Management Agency – Chennai - 5 Additional Cheif Secretary & Commissioner 044-28523299 9445000444 of Revenue Administration, Chennai -5 044-28414513, Disaster Management, Chennai 044-1070 Control Room 044-28414512 Emergency Toll Free Numbers Disaster Rescue, 1077 District Collector Office, Thanjavur Child Line 1098 Police 100 Fire & Rescue Department 101 Medical Helpline 104 Ambulance 108 Women’s Helpline 1091 National Highways Emergency Help 1033 Old Age People Helpline 1253 Coastal Security 1718 Blood Bank 1910 Eye Donation 1919 Railway Helpline 1512 AIDS Helpline 1097 2 Meteorological Research Centre S. -

Dried Chilli Pepppers Postsession For
ECE/TRADE/C/WP.7/GE.2/2011/INF.9 23 June 2011 Post session July 2011 Economic Commission for Europe Committee on Trade Working Party on Agricultural Quality Standards Specialized Section on Standardization of Dry and Dried Produce Fifty- eight session Geneva, 27-30 June 2011 Item 6 (a) of the provisional agenda New UNECE Standards Comments Submitted by Mexico/June 2011 This document has been prepared following the decision of the Working Party to initiate work on a new standard for whole dried chilli peppers (ECE/TRADE/C/WP.7/2007/27, paragraph 32). It is the revised version of document ECE/TRADE/C/WP.7/2008/4. Suggested revisions are indicated by strikethrough/underline. POST SESSION DOCUMENT JULY 2011 I. Definition of produce This Standard applies to whole dried chilli peppers of varieties (cultivars) grown from Capsicum annuum L., intended for direct consumption or for food when intended to be mixed with other products for direct consumption without further processing. This standard does not apply to whole dried chilli peppers for industrial processing.1 This standard covers the following commercial types of whole dried chilli peppers Commercial types of whole dried chi lli peppers include : ancho, de árbol, guajillo, mulato, pasilla and puya 2. II. Provisions concerning quality The purpose of the standard is to define the quality requirements of whole dried chilli peppers at the export-control stage, after preparation and packaging. 1 For the correct application of this Standard, see other definitions contained in annex I . 2 For the correct understanding of the levels of pungency (intensity) see annex III. -

The Great Author of Summaries- Contemporary of Buddhaghosa
UNIVERSITY OF CEYLON REVIEW The Great Author of Summaries- Contemporary of Buddhaghosa UDDHADATTA'S Manuals of Vinaya and Abhidhamma are now known to orientalists through the editions of the Pali Text B Society. I In the Introductory Note to the Abhidhammavatiira, Mrs. C. A. F. Rhys Davids remarks: "According to the legend, Buddhadatta recast in a condensed shape that which Buddhaghosa handed on, in Pali, from the Sinhalese Commentaries. But the psychology and philosophy are pre- sented through the prism of a second vigorous intellect, under fresh aspects, in a style often less discursive and more graphic than that of the great Com- msntator, and with strikingly rich vocabulary, as is revealed by the dimensions of the Index. .. So little was the modestly expressed ambition of Buddha- datta's Commentator realized :-that the great man's death would leave others room to emerge from eclipse-a verse that forcibly recalls George Meredith's quip uttered at Tennyson's funeral-' Well, a -, it's a great day for the minor poets'!" The legend that she refers to is found in the Pali Commentary on the Vina- yavinicchaya and in the Buddhaghosu-p paiti, The former work was composed by the Sinhalese Elder, Mahasami Vacissara, in about the r zth century A.D., and is still unpublished. In its introduction it states: "Ayarn kira Bhadanta- Buddhadatt acariyo Lankadlpato sajatibhfimin Jambudipam agacchanto Bhadanta-Buddhaghosacariyau J ambudipavasikehi . mahatheravarehi kataradhanan Sihalatthakathan parivattetva . miilabhasaya tipitaka- pariyattiya atthakatham likhitum Lankadlpam gacchantam antar amagge disva sakacchaya samupaparikkhitva ... balavaparitosam patva ... 'tumhe yathadhippeta-pariyantarn likhitam atthakatham amhakam pesetha, mayam assa pan a .. -
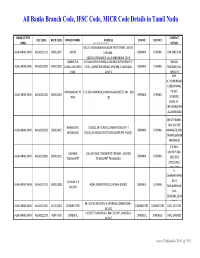
Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu
All Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu NAME OF THE CONTACT IFSC CODE MICR CODE BRANCH NAME ADDRESS CENTRE DISTRICT BANK www.Padasalai.Net DETAILS NO.19, PADMANABHA NAGAR FIRST STREET, ADYAR, ALLAHABAD BANK ALLA0211103 600010007 ADYAR CHENNAI - CHENNAI CHENNAI 044 24917036 600020,[email protected] AMBATTUR VIJAYALAKSHMIPURAM, 4A MURUGAPPA READY ST. BALRAJ, ALLAHABAD BANK ALLA0211909 600010012 VIJAYALAKSHMIPU EXTN., AMBATTUR VENKATAPURAM, TAMILNADU CHENNAI CHENNAI SHANKAR,044- RAM 600053 28546272 SHRI. N.CHANDRAMO ULEESWARAN, ANNANAGAR,CHE E-4, 3RD MAIN ROAD,ANNANAGAR (WEST),PIN - 600 PH NO : ALLAHABAD BANK ALLA0211042 600010004 CHENNAI CHENNAI NNAI 102 26263882, EMAIL ID : CHEANNA@CHE .ALLAHABADBA NK.CO.IN MR.ATHIRAMIL AKU K (CHIEF BANGALORE 1540/22,39 E-CROSS,22 MAIN ROAD,4TH T ALLAHABAD BANK ALLA0211819 560010005 CHENNAI CHENNAI MANAGER), MR. JAYANAGAR BLOCK,JAYANAGAR DIST-BANGLAORE,PIN- 560041 SWAINE(SENIOR MANAGER) C N RAVI, CHENNAI 144 GA ROAD,TONDIARPET CHENNAI - 600 081 MURTHY,044- ALLAHABAD BANK ALLA0211881 600010011 CHENNAI CHENNAI TONDIARPET TONDIARPET TAMILNADU 28522093 /28513081 / 28411083 S. SWAMINATHAN CHENNAI V P ,DR. K. ALLAHABAD BANK ALLA0211291 600010008 40/41,MOUNT ROAD,CHENNAI-600002 CHENNAI CHENNAI COLONY TAMINARASAN, 044- 28585641,2854 9262 98, MECRICAR ROAD, R.S.PURAM, COIMBATORE - ALLAHABAD BANK ALLA0210384 641010002 COIIMBATORE COIMBATORE COIMBOTORE 0422 2472333 641002 H1/H2 57 MAIN ROAD, RM COLONY , DINDIGUL- ALLAHABAD BANK ALLA0212319 NON MICR DINDIGUL DINDIGUL DINDIGUL -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2013 [Price: Rs. 54.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 41] CHENNAI, WEDNESDAY, OCTOBER 23, 2013 Aippasi 6, Vijaya, Thiruvalluvar Aandu–2044 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 2893-3026 Notice .. 3026-3028 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 43888. My son, D. Ramkumar, born on 21st October 1997 43891. My son, S. Antony Thommai Anslam, born on (native district: Madurai), residing at No. 4/81C, Lakshmi 20th March 1999 (native district: Thoothukkudi), residing at Mill, West Colony, Kovilpatti, Thoothukkudi-628 502, shall Old No. 91/2, New No. 122, S.S. Manickapuram, Thoothukkudi henceforth be known as D. RAAMKUMAR. Town and Taluk, Thoothukkudi-628 001, shall henceforth be G. DHAMODARACHAMY. known as S. ANSLAM. Thoothukkudi, 7th October 2013. (Father.) M. v¯ð¡. Thoothukkudi, 7th October 2013. (Father.) 43889. I, S. Salma Banu, wife of Thiru S. Shahul Hameed, born on 13th September 1975 (native district: Mumbai), 43892. My son, G. Sanjay Somasundaram, born residing at No. 184/16, North Car Street, on 4th July 1997 (native district: Theni), residing Vickiramasingapuram, Tirunelveli-627 425, shall henceforth at No. 1/190-1, Vasu Nagar 1st Street, Bank be known as S SALMA. -

Silver Heights Farm
SILVER HEIGHTS FARM Certified Organic, Open Pollinated Unusual & Heirloom Vegetable Plants DEDICATED TO THE PRESERVATION OF HEIRLOOM PLANTS 2009 Catalog Featuring varieties to dazzle the gardener’s imagination Multitudes of Tomatoes, Tomatillos, & Ground Cherries Peppers & Eggplants galore Glorious Cucumbers, Gherkins, & melons Other funky vegetables 1 NURSERY HOURS April By chance, best from 10-12, 1-3. Closed Sunday. May & June Friday – Saturday, 10-4; Sunday, 10-2; other days by chance July Friday- Saturday, 10-3; other days by chance . Closed Sunday. August – mid-October By chance, best from 10-12, 1-3. Closed Sunday. There is NO PHONE service at the nursery – no land lines and no cell service. Instead, the phone rings at Trina’s home, 10 miles away. Inconvenient, we know, but we are in the “boonies.” Payment is by cash, check and money orders only, as we do not accept any credit cards. Sorry, we do not ship or deliver. All prices are subject to change after June 1, 2009. There are many more vegetable varieties at the nursery than in the catalog. Also, we have an incredible selection of herbs and flowers. We look forward to your visit. WE MOVED in 2005 – NEW LOCATION The nursery moved in 2005 away from Trina’s backyard. The nursery for Silver Heights Farm is now located at the Gorzynski Farm at 7381 State Route 52 in Cochecton Center. The nursery is at the front of the fields, next to the big grey block 4-storied barn with a red roof. Parking is alongside Route 52. Please do not park in front of the barn or in the driveway, as the Gorzynski Farm folks need that space for their operation. -

TNEB LIMITED TANGEDCO TANTRANSCO BULLETIN December
1 TNEB LIMITED TANGEDCO TANTRANSCO BULLETIN December – 2018 CONTENTS Page No 1. PART – I NEWS & NOTES … … … 2 2. PART – II GENERAL ADMINISTRATIVE & SERVICES … … … 8 3. PART – III FINANCE … … … 21 4. PART – IV TECHNICAL … … … 33 5. INDEX … … … 55 6. CONSOLIDATED INDEX … … … 59 A request With the present issue of the TANGEDCO Bulletin for December 2018 Volume XXXVII (37) which completed. The recipients of the Bulletin are request to have the 12 issues of Volume XXXVII bound in one part from January 2018 to December 2018. A consolidated Index for volume XXXVII has been included in this issue for reference. 2 NEWS & NOTES PART – I I. GENERATION/RELIEF PARTICULARS:- The Generation/Relief particulars for the month of December 2018 were as follows: Sl.No Particulars In Million Units I. TNEB GENERATION (Gross) Hydro 488.582 Thermal 2318.235 Gas 145.094 Wind 0.100 TNEB TOTAL 2952.011 II. NETT PURCHASES FROM CGS 2730.033 III. PURCHASES IPP 221.921 Windmill Private 243.604 CPP, Co- generation & Bio-Mass (Provisional) 16.500 Solar (Private) 274.640 Through Traders (nett purchase) 1758.316 TOTAL PURCHASES 2514.981 IV. Total Wheeling Quantum by HT consumers 702.424 Total Wheeling Quantum to Other States by Pvt. Generators 11.053 Total TNEB Power generation for sale 0.000 TOTAL WHEELING 713.477 Power Sale by TANGEDCO (Exchange) 0.000 Power Sale by TANGEDCO (STOA under Bilateral) 0.000 Power Sale by Private Generators (Exchange) (-)8.403 Power Sale by Private Generators (Bilateral) (-)2.650 Power balance under SWAP 2.688 V. TOTAL (TNEB Own Gen + Purchase + wheeling quantum + SWAP) 8902.138 VI. -

2020 Hugo Feed Mill Pepper List Type Description
2020 Hugo Feed Mill www.hugofeedmill.com Pepper List 651-429-3361 New Name Type Description 09154 Hot A long, skinny Thai ¼" x 2" red pepper. Grows in clusters pointing upwards. 2018 7 Pot B. Gum X Pimenta de Neyde Hot Fiery Hot with a Bleeding Stem. Salmon to Red skin with ocassional purple blush. 7 Pot Brain Strain Hot Scorching hot, fruity flavored peppers. High yield. Said by some to be the hottest of the 7 Pot family. 7 Pot Brain Strain Yellow Hot Yellow version of the Red Brain Strain but less heat Originally from Trinidad, pineapple flavor, w/ 7 Pot heat 7 Pot Bubble Gum Hot Super Hot w/ floral smell and fruity undertones Red fruit w/ stem and cap ripening to bubblegum color 7 Pot Bubble Gum* Hot Super Hot w/ floral smell and fruity undertones Red fruit w/ stem and cap ripening to bubblegum color 7 Pot Lava Brown Hot Another Pepper in the Super Hots. Heat is remarkable Smokey fruity, brown pepper with the scorpion look 7 Pot Lave Brown Variant Red Hot Moruga Scorpion X 7 Pot Primo cross Morurga look with a stinger, extremely hot 2020 Aji Amarillo Hot 4-5" long pepper. Deep yellow/orange. Fruity flavor with intense heat. Aji Perrana Hot Large Aji type orange pepper from Peru. Similar to an Aji Amarillo but smaller. Aji Chombo Hot Robust rounded red scorcher from Panama. Scotch bonnet type fruit w/ sweet flavor then BAM! Aji Cito Hot Awesome producer of beautiful torpedo shaped peppers. Peruvian pepper, with 100,000 SHU and a hint of citrus. -

Download Download
The Journal of Threatened Taxa (JoTT) is dedicated to building evidence for conservaton globally by publishing peer-reviewed artcles OPEN ACCESS online every month at a reasonably rapid rate at www.threatenedtaxa.org. All artcles published in JoTT are registered under Creatve Commons Atributon 4.0 Internatonal License unless otherwise mentoned. JoTT allows unrestricted use, reproducton, and distributon of artcles in any medium by providing adequate credit to the author(s) and the source of publicaton. Journal of Threatened Taxa Building evidence for conservaton globally www.threatenedtaxa.org ISSN 0974-7907 (Online) | ISSN 0974-7893 (Print) Communication Vaduvur and Sitheri lakes, Tamil Nadu, India: conservation and management perspective V. Gokula & P. Ananth Raj 26 May 2021 | Vol. 13 | No. 6 | Pages: 18497–18507 DOI: 10.11609/jot.5547.13.6.18497-18507 For Focus, Scope, Aims, and Policies, visit htps://threatenedtaxa.org/index.php/JoTT/aims_scope For Artcle Submission Guidelines, visit htps://threatenedtaxa.org/index.php/JoTT/about/submissions For Policies against Scientfc Misconduct, visit htps://threatenedtaxa.org/index.php/JoTT/policies_various For reprints, contact <[email protected]> The opinions expressed by the authors do not refect the views of the Journal of Threatened Taxa, Wildlife Informaton Liaison Development Society, Zoo Outreach Organizaton, or any of the partners. The journal, the publisher, the host, and the part- Publisher & Host ners are not responsible for the accuracy of the politcal boundaries shown in the maps by the authors. Member Threatened Taxa Journal of Threatened Taxa | www.threatenedtaxa.org | 26 May 2021 | 13(6): 18497–18507 ISSN 0974-7907 (Online) | ISSN 0974-7893 (Print) OPEN ACCESS htps://doi.org/10.11609/jot.5547.13.6.18497-18507 #5547 | Received 11 November 2019 | Final received 17 April 2021 | Finally accepted 05 May 2021 COMMUNICATION Vaduvur and Sitheri lakes, Tamil Nadu, India: conservaton and management perspectve V. -

Product-Range-Westla
Product range westlandpeppers Fresh chilies and bell peppers Dried chilies Sauces Spices and herbs Organic products About Westlandpeppers OUR COMPANY In 1930 Hendrik Boekestijn Westlandpeppers is located At our main location in De started the company that’s at two locations in Lier, we have a large now known as 'Westland', the main location packaging hall where all our Westlandpeppers. He is in De Lier and a second products are packed. started with the cultivation location in ‘s-Gravenzande. Westlandpeppers offers of grapes, peaches, leeks With totally 8,6 hectare of various kilo- and small and cauliflowers. Later he greenhouses. From April till packages. The packages was succeeded by his son November the products come can be delivered with one or Pieter Boekestijn. from our own greenhouses in more products, according to Meanwhile, his three sons the Netherlands and from your own composition. For Henk, Dolf and Pieter have November till April the the small packages, the taken over the company and products come from Spain, customer can choose from a they cultivate a wide range Morocco and Israel, where large number of chilies and snack we work with the same of possibilities, but an peppers. In the meantime, growers for years. This individual interpretation or a the fourth generation works allows us to offer chilies and specific design is also at the company as well. snack peppers year-round. possible. Fresh Assortment Spanish chili / Cayenne peper The Spanish chilli, also known as the 'common' pepper or cayenne pepper, is perhaps the most widely used pepper worldwide. Spanish or red/green/yellow/orange pepper is a collective name for the well-known elongated chilli peppers. -

Hot Chili Chili Tool Oa., Styrke & Scoville – 3 – Rev
Hot Chili Chili tool oa., Styrke & Scoville – 3 – rev. 1.2 Stor liste med relativ styrke og Scoville værdi (..eller den lille gruppe oversigt med kendte chili) Styrke Navne Scoville Noter ▪ Ren Capsaicin 16,000,000 12 ▪ Carolina Reaper 2,200,000 ▪ Trinidad Moruga Scorpion 2,009,231 ▪ ▪ 7 Pot Douglah 1,853,396 10++++ til 12 ▪ Trinidad Scorpion Butch T 1,463,700 ▪ Naga Viper Pepper 1,382,118 ▪ 7 Pot Barrackpore 1,300,000 ▪ 7 Pot Jonah 1,200,000 ▪ 7 Pot Primo 1,200,000 ▪ New Mexico Scorpion 1,191,595 ▪ Infinity Chili 1,176,182 ▪ Bedfordshire Super Naga 1,120,000 ▪ ▪ Dorset Naga chili 1,100,000 ▪ Naga Jolokia 1,100,000 ▪ Naga Morich 1,100,000 ▪ Spanish Naga Chili 1,086,844 ▪ 7 Pot Madballz 1,066,882 ▪ Bhut Jolokia chili 1,041,427 ▪ Chocolate Bhut Jolokia 1,001,304 ▪ 7 Pot Brain Strain 1,000,000 ▪ Bhut Jolokia Indian Carbon 1,000,000 ▪ Trinidad Scorpion 1,000,000 ▪ Raja Mirch 900,000 ▪ Habanaga chili 800,000 ▪ Nagabon Jolokia 800,000 ▪ Red Savina Habanero 580,000 10++++ Chili Home Side 1/12 Hot Chili Chili tool oa., Styrke & Scoville – 3 – rev. 1.2 10+++ og 10++++ ▪ Fatalii 500,000 10++++ ▪ Aji Chombo 500,000 ▪ Pingo de Ouro 500,000 ▪ Aribibi Gusano 470,000 ▪ Caribbean Red Habanero 400,000 ▪ Chocolate Habanero 350,000 10++++ ▪ Datil chili 350,000 10++++ ▪ Habanero 350,000 ▪ Jamaican Hot chili 350,000 ▪ Madame Jeanette chili 350,000 ▪ Rocoto chili 350,000 ▪ Scotch Bonnet 350,000 10+++ ▪ Zimbabwe Bird Chili 350,000 ▪ Adjuma 350,000 ▪ Guyana Wiri Wiri 350,000 10+++ ▪ Tiger Paw 348,634 ▪ Big Sun Habanero 325,000 ▪ Orange Habanero 325,000 9 og 10+++ ▪ Mustard Habanero 300,000 ▪ Devil’s Tongue 300,000 10++++ ▪ Orange Rocoto chili 300,000 10++ ▪ Paper Lantern Habanero 300,000 ▪ Piri Piri 300,000 8-10 ▪ Red Cheese 300,000 ▪ Red Rocoto 300,000 10++ ▪ Tepin chili 300,000 ▪ Thai Burapa 300,000 ▪ White Habanero 300,000 9-10 ▪ Yellow Habanero 300,000 ▪ Texas Chiltepin 265,000 ▪ Pimenta de Neyde 250,000 ▪ Maori 240,000 ▪ Quintisho 240,000 Chili Home Side 2/12 Hot Chili Chili tool oa., Styrke & Scoville – 3 – rev. -

3. Annual Quality Assurance Report
Annual Quality Assurance Report (AQAR 2017 - 18) Submitted to NATIONAL ASSESSMENT AND ACCREDITATION COUNCIL Bengal u ru – 560 072. By Internal Quality Assurance Cell (IQAC) SENGAMALA THAYAAR EDUCATIONAL TRUST WOMEN’S COLLEGE (Affiliated to Bharathidasan University, Tiruchirappalli) (NAAC accredited ‘A’ Grade with CGPA 3.45 / 4.00) SUNDARAKKOTTAI, MANNARGUDI – 614 016 TIRUVARUR DISTRICT, TAMIL NADU. AQAR 2017-18 – NAAC – Bengaluru The Annual Quality Assurance Report (AQAR) of the IQAC Part – A AQAR for the year 2017-2018 1. Details of the Institution 1.1 Name of the Institution SENGAMALA THAYAAR EDUCATIONAL TRUST WOMEN’S COLLEGE 1.2 Address Line 1 Main Road Address Line 2 Sundarakkottai Village City/Town Mannargudi State Tamil Nadu Pin Code 614 016 Institution e-mail address [email protected] / [email protected] Contact Nos. 04367-255423 Name of the Head of Dr.S.Amudha, M. Com., M.Phil., M.B.A., Ph.D. the Institution Tel. No. with STD Code 04367- 255405 Mobile 9443703331 Name of the IQAC Dr. R. Saravanamuthu M.Sc., M.Phil., Ph.D. Co-ordinator STET. Women’s College, Sundarakkottai, Mannargudi, TN, India Page 1 AQAR 2017-18 – NAAC – Bengaluru Mobile 9842618181 [email protected] IQAC e-mail address 1.3 NAAC Track ID TNCOGN19382 OR 1.4 NAAC Executive EC (SC)/05/A&A/085 dated 03.03.2015 Committee No. & Date 1.5 Website address www.stet.edu.in Web-link of the AQAR http://stet.edu.in/AQAR /AQAR17-18.pdf 1.6 Accreditation Details YEAR OF VALIDITY Sl. No. CYCLE GRADE CGPA ACCREDITATION PERIOD 1 1st Cycle A 3.45 03.03.2015 02.03.2020 2 2nd Cycle 3 3rd Cycle 4 4th Cycle 1.7 Date of Establishment of IQAC: DD/MM/YYYY 20.06.2011 1.8 Details of the previous year’s AQAR submitted to NAAC after the latest Assessment and Accreditation by NAAC i.