Tg-Pesticides-E.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ri Wkh% Lrorjlfdo (Iihfwv Ri 6Hohfwhg &Rqvwlwxhqwv
Guidelines for Interpretation of the Biological Effects of Selected Constituents in Biota, Water, and Sediment November 1998 NIATIONAL RRIGATION WQATER UALITY P ROGRAM INFORMATION REPORT No. 3 United States Department of the Interior Bureau of Reclamation Fish and Wildlife Service Geological Survey Bureau of Indian Affairs 8QLWHG6WDWHV'HSDUWPHQWRI WKH,QWHULRU 1DWLRQDO,UULJDWLRQ:DWHU 4XDOLW\3URJUDP LQIRUPDWLRQUHSRUWQR *XLGHOLQHVIRU,QWHUSUHWDWLRQ RIWKH%LRORJLFDO(IIHFWVRI 6HOHFWHG&RQVWLWXHQWVLQ %LRWD:DWHUDQG6HGLPHQW 3DUWLFLSDWLQJ$JHQFLHV %XUHDXRI5HFODPDWLRQ 86)LVKDQG:LOGOLIH6HUYLFH 86*HRORJLFDO6XUYH\ %XUHDXRI,QGLDQ$IIDLUV 1RYHPEHU 81,7('67$7(6'(3$570(172)7+(,17(5,25 %58&(%$%%,776HFUHWDU\ $Q\XVHRIILUPWUDGHRUEUDQGQDPHVLQWKLVUHSRUWLVIRU LGHQWLILFDWLRQSXUSRVHVRQO\DQGGRHVQRWFRQVWLWXWHHQGRUVHPHQW E\WKH1DWLRQDO,UULJDWLRQ:DWHU4XDOLW\3URJUDP 7RUHTXHVWFRSLHVRIWKLVUHSRUWRUDGGLWLRQDOLQIRUPDWLRQFRQWDFW 0DQDJHU1,:43 ' %XUHDXRI5HFODPDWLRQ 32%R[ 'HQYHU&2 2UYLVLWWKH1,:43ZHEVLWHDW KWWSZZZXVEUJRYQLZTS Introduction The guidelines, criteria, and other information in The Limitations of This Volume this volume were originally compiled for use by personnel conducting studies for the It is important to note five limitations on the Department of the Interior's National Irrigation material presented here: Water Quality Program (NIWQP). The purpose of these studies is to identify and address (1) Out of the hundreds of substances known irrigation-induced water quality and to affect wetlands and water bodies, this contamination problems associated with any of volume focuses on only nine constituents or the Department's water projects in the Western properties commonly identified during States. When NIWQP scientists submit NIWQP studies in the Western United samples of water, soil, sediment, eggs, or animal States—salinity, DDT, and the trace tissue for chemical analysis, they face a elements arsenic, boron, copper, mercury, challenge in determining the sig-nificance of the molybdenum, selenium, and zinc. -

The Decomposition Kinetics of Peracetic Acid and Hydrogen Peroxide in Municipal Wastewaters
Disinfection Forum No 10, October 2015 The Decomposition Kinetics of Peracetic Acid and Hydrogen Peroxide in Municipal Wastewaters INTRODUCTION Efficient control of microbial populations in municipal wastewater using peracetic acid (PAA) requires an understanding of the PAA decomposition kinetics. This knowledge is critical to ensure the proper dosing of PAA needed to achieve an adequate concentration within the contact time of the disinfection chamber. In addition, the impact of PAA on the environment, post-discharge into the receiving water body, also is dependent upon the longevity of the PAA in the environment, before decomposing to acetic acid, oxygen and water. As a result, the decomposition kinetics of PAA may have a significant impact on aquatic and environmental toxicity. PAA is not manufactured as a pure compound. The solution exists as an equilibrium mixture of PAA, hydrogen peroxide, acetic acid, and water: ↔ + + Acetic Acid Hydrogen Peroxide Peracetic Acid Water PeroxyChem’s VigorOx® WWT II Wastewater Disinfection Technology contains 15% peracetic acid by weight and 23% hydrogen peroxide as delivered. Although hydrogen peroxide is present in the formulation, peracetic acid is considered to be the active component for disinfection1 in wastewater. There have been several published studies investigating the decomposition kinetics of PAA in different water matrices, including municipal wastewater2-7. Yuan7 states that PAA may be consumed in the following three competitive reactions: 1. Spontaneous decomposition 2 CH3CO3H à 2 CH3CO2H + O2 Eq (1) 2. Hydrolysis CH3CO3H + H2O à CH3CO2H + H2O2 Eq (2) 3. Transition metal catalyzed decomposition + CH3CO3H + M à CH3CO2H + O2 + other products Eq (3) At neutral pH’s, both peracetic acid and hydrogen peroxide can be rapidly consumed by these reactions7 (hydrogen peroxide will decompose to water and oxygen via 2H2O2 à 2H2O + O2). -

TOXICOLOGICAL PROFILE for HEPTACHLOR and HEPTACHLOR EPOXIDE
TOXICOLOGICAL PROFILE FOR HEPTACHLOR and HEPTACHLOR EPOXIDE Prepared by: Syracuse Research Corporation Under Contract No. 200-2004-09793 Prepared for: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry November 2007 HEPTACHLOR AND HEPTACHLOR EPOXIDE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. HEPTACHLOR AND HEPTACHLOR EPOXIDE iii UPDATE STATEMENT A Toxicological Profile for Heptachlor/Heptachlor Epoxide, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 HEPTACHLOR AND HEPTACHLOR EPOXIDE iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. -

Peracetic Acid Processing
Peracetic Acid Processing Identification Chemical Name(s): CAS Number: peroxyacetic acid, ethaneperoxic acid 79-21-0 Other Names: Other Codes: per acid, periacetic acid, PAA NIOSH Registry Number: SD8750000 TRI Chemical ID: 000079210 UN/ID Number: UN3105 Summary Recommendation Synthetic / Allowed or Suggested Non-Synthetic: Prohibited: Annotation: Synthetic Allowed (consensus) Allowed only for direct food contact for use in wash water. Allowed as a (consensus) sanitizer on surfaces in contact with organic food. (consensus) From hydrogen peroxide and fermented acetic acid sources only. (Not discussed by processing reviewers--see discussion of source under Crops PAA TAP review.) Characterization Composition: C2H4O3. Peracetic acid is a mixture of acetic acid (CH3COOH) and hydrogen peroxide (H2O2) in an aqueous solution. Acetic acid is the principle component of vinegar. Hydrogen peroxide has been previously recommended by the NOSB for the National List in processing (synthetic, allowed at Austin, 1995). Properties: It is a very strong oxidizing agent and has stronger oxidation potential than chlorine or chlorine dioxide. Liquid, clear, and colorless with no foaming capability. It has a strong pungent acetic acid odor, and the pH is acid (2.8). Specific gravity is 1.114 and weighs 9.28 pounds per gallon. Stable upon transport. How Made: Peracetic acid (PAA) is produced by reacting acetic acid and hydrogen peroxide. The reaction is allowed to continue for up to ten days in order to achieve high yields of product according to the following equation. O O || || CH3-C-OH + H2O2 CH3C-O-OH + H2O acetic acid hydrogen peroxyacetic peroxide acid Due to reaction limitations, PAA generation can be up to 15% with residual levels of hydrogen peroxide (up to 25%) and acetic acid (up to 35%) with water up to 25%. -

Mirex Group: 04
DaMocles WiSe 19/20: Mirex Group: 04 Marius Wollrab Milan Zengeler Maurice Wingsheim February 2, 2020 Contents 1. General information 1 2. Synthesis 2 3. Toxicology 2 4. Ban 3 II. References I I 1. General information Mirex, Dechlorane or Perchloropentacyclodecane (figure 1) is a synthetic produced, white, odorless, crystalline solid chlorinated hydrocarbon. The IUPAC name is: 1,1a,2,2,3,3a,4,5,5,5a,5b,6- dodecachlorooctahydro-1H-1,3,4-(methanetriyl)cyclobuta[cd]pentalene and the GHS classification is depicted in figure 2. Figure 1: Structure of Mirex [1] Figure 2: GHS Classification [2] 1 Chemical and physical properties: Property name Property value 1 Molecular weight 545,5 g mol− 1 Exact mass 545,617 382 g mol− Melting point 485 ◦C Chemical formula C10Cl12 CAS-Nr. 2385-85-5 Mirex was commonly used as a stomach insecticide against imported fire ants Solenopsis saevissima richteri and as a fire-retardant for plastics, paper, electronics and paint. As a fire-retardant, Mirex was sold underthe name of Dechlorane. The top producer of Mirex was the USA and up to 75% were exported to country’s in south and middle America.[2] 2. Synthesis Mirex can be synthesized by dimerization of hexachlorocyclopentadiene (figure 3). For this reaction aluminiumchloride is used as catalyst. Figure 3: Synthesis of Mirex 3. Toxicology Mirex targets and damages the liver, kidney, eye, thyroid, central nervous system and the reproductive organs. Studies on laboratory animals have caused it to be classified as a possible human carcinogen.[3] 2 Mirex is also dangerous for humans and the environment, because of the risk of bioaccumulation. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Peracetic Acid
Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products Evaluation of active substances Assessment Report Peracetic acid Product-types 11 and 12 (Preservatives for liquid cooling and processing systems) (Slimicides) August 2016 Finland Peracetic acid Product-types 11 and 12 August 2016 CONTENTS 1. STATEMENT OF SUBJECT MATTER AND PURPOSE ........................................................ 2 1.1. Procedure followed ............................................................................................. 2 1.2. Purpose of the assessment report ....................................................................... 2 2. OVERALL SUMMARY AND CONCLUSIONS ..................................................................... 4 2.1. Presentation of the Active Substance .................................................................. 4 2.1.1. Identity, Physico-Chemical Properties & Methods of Analysis ................. 4 2.1.2. Intended Uses and Efficacy ..................................................................... 7 2.1.3. Classification and Labelling ..................................................................... 8 2.2. Summary of the Risk Assessment ....................................................................... 9 2.2.1. Human Health Risk Assessment .............................................................. 9 2.2.1.1. Hazard identification ................................................................................... 9 2.2.1.2. Effects assessment ................................................................................... -

Particularly Hazardous Substances
Particularly Hazardous Substances In its Laboratory Standard, OSHA requires the establishment of additional protections for persons working with "Particularly Hazardous Substances" (PHS). OSHA defines these materials as "select" carcinogens, reproductive toxins and acutely toxic materials. Should you wish to add: explosive, violently reactive, pyrophoric and water-reactve materials to this category, the information is included. Carbon nanotubes have also been added due to their suspected carcinogenic properties. This table is designed to assist the laboratory in the identification of PHS, although it is not definitively conclusive or entirely comprehensive. *Notes on the proper use of this table appear on page 12. 1 6 5 2 3 4 Substance CAS National Toxicity National Program Carcinogen Toxin Acute Regulated OSHA Carcinogen Group IARC Carcinogen Toxin Reproductive Violently Reactive/ Explosive/Peroxide Forming/Pyrophoric A-a-C(2-Amino-9H-pyrido[2,3,b]indole) 2648-68-5 2B Acetal 105-57-7 yes Acetaldehyde 75-07-0 NTP AT 2B Acrolein (2-Propenal) 107-02-8 AT Acetamide 126850-14-4 2B 2-Acetylaminofluorene 53-96-3 NTP ORC Acrylamide 79-06-6 NTP 2B Acrylyl Chloride 814-68-6 AT Acrylonitrile 107-13-1 NTP ORC 2B Adriamycin 23214-92-8 NTP 2A Aflatoxins 1402-68-2 NTP 1 Allylamine 107-11-9 AT Alkylaluminums varies AT Allyl Chloride 107-05-1 AT ortho-Aminoazotoluene 97-56-3 NTP 2B para-aminoazobenzene 60-09-3 2B 4-Aminobiphenyl 92-67-1 NTP ORC 1 1-Amino-2-Methylanthraquinone 82-28-0 NTP (2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole) 67730-11-4 2B -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Persistent Organic Pollutants
PERSISTENT ORGANIC POLLUTANTS An Assessment Report on: DDT-Aldrin-Dieldrin-Endrin-Chlordane Heptachlor-Hexachlorobenzene Mirex-Toxaphene Polychlorinated Biphenyls Dioxins and Furans Prepared by: L. Ritter, K.R. Solomon, J. Forget Canadian Network of Toxicology Centres 620 Gordon Street Guelph ON Canada and M. Stemeroff and C.O'Leary Deloitte and Touche Consulting Group 98 Macdonell St., Guelph ON Canada For: The International Programme on Chemical Safety (IPCS) within the framework of the Inter-Organization Programme for the Sound Management of Chemicals (IOMC) This report is produced for the International Programme on Chemical Safety (IPCS). The work is carried out within the framework of the Inter-Organization Programme for the Sound Management of Chemicals (IOMC). The report does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of human resources in the field of chemical safety. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, strengthening capabilities for prevention of an response to chemical accidents and their follow-up, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. -

Peracetic Acid Bleaching CH CO H
Peracetic Acid Bleaching CH3CO3H Introduction of Bleaching Bleaching is a chemical decoloration and delignification process carried out on various types of pulp. DliDelignifi cati on Removal of the chromophores At in dus tr ia l scal e, bl eachi ng i s perf ormed b y chl ori ne, chl ori ne dioxide, oxygen, hydrogen peroxide, ozone and peracetic acid. Effic iency: ClO2 = Cl2 = O3 >CH3COOOH > H2O2 >O> O2 Often bleaching chemicals are wasted in secondary reactions. D. Lachenal and C. Chirat, Cellulose Chem. Technol., 39, 5-6, 511-156 (2005) 2 Electron Exchange Theory Ring opening of the aromatic units necessitates the exchange of 4 e - # of e- can be exchanged per bleaching molecule - + O3 + 6 e + 6 H → 3 H2O - + - CCOlO2 + 55e e + 4 H → Cl + 2 H2O2 - + O2 + 4 e + 4 H → 2 H2O - + H2O2 + 2 e + 2 H → 2 H2O - + CH3COOOH + 2 e + 2 H → CH3COOH + H2O # of e- exchanged per molecule decreases in the following order: O3 > ClO2 > O2 > H2O2 = CH3COOOH D. Lachenal and C. Chirat, Cellulose Chem. Technol., 39, 5-6, 511-156 (2005) 3 Actual Electron Exchange - ClO2 and O3 100 g un bleac he d SW k raf t pul p, k appa # f rom 30 to ~ 4 -5 - 2.3% ClO2 (0.034 mole) on pulp: 0.034 x 5 = 0.17 e is exchanged - 14%1.4% O3 (0.03 mole)on pulp: 0 .03 x 6 = 018e0.18 e is exchanged Chemicals % consumed1 Kappa number * ClO2 2.3 4.5 O3 1.5 3.5 O3 1.4 5.0 O3 1.3 6.5 1Chemicals consumed are shown as % on pulp. -
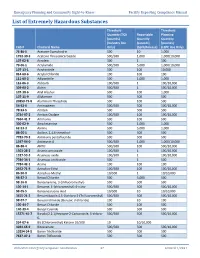
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500