Mechanical Stress Induce PG-E2 in Murine Synovial Fibroblasts Originating from the Temporomandibular Joint
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
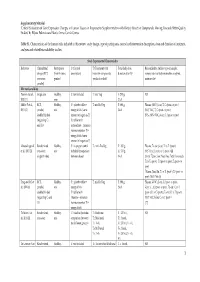
Critical Evaluation of Gene Expression Changes in Human Tissues In
Supplementary Material ‘Critical Evaluation of Gene Expression Changes in Human Tissues in Response to Supplementation with Dietary Bioactive Compounds: Moving Towards Better-Quality Studies’ by Biljana Pokimica and María-Teresa García-Conesa Table S1. Characteristics of the human trials included in this review: study design, type of participants, control and intervention description, dose and duration of treatment, analyses and related bioavailability studies. Study Experimental Characteristics Reference Clinical trial Participants C (Control T (Treatment with Total daily dose, Bioavailability studies: type of sample, design (RCT, (health status, description) bioactive compounds, duration (d or h)1 compounds and (or) metabolites analysed, crossover, gender) products or diet) main results2 parallel) Mix meals and diets Persson I et al., Single arm Healthy, C: not included T: mix Veg T: 250 g, NR 2000 [1] men 21 d Møller P et al., RCT, Healthy, C1: placebo tablet + T: mix FruVeg T: 600 g, Plasma: (NS↑) β-car, T, C2 (post- vs pre-) 2003 [2] parallel, mix energy drink (same 24 d (NC) VitC, T, C2 (post- vs pre-) double blinded amount of sugars as T) (NS↓, 69%) VitC, β-car, C1 (post- vs pre-) (regarding C1 C2: tablet with and C2) antioxidants + minerals (same amount as T) + energy drink (same amount of sugars as T) Almendingen K Randomized, Healthy, C: no proper control T1,2: mix FruVeg T1: 300 g, Plasma: ↑α-car, β-car, T2 vs T1 (post-) et al., 2005 [3] crossover, mix included (comparison T2: 750 g, (NS↑) Lyc, Lut, T2 vs T1 (post-) [4] single -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Characterization of Visceral and Subcutaneous Adipose Tissue
J. Perinat. Med. 2016; 44(7): 813–835 Shali Mazaki-Tovi*, Adi L. Tarca, Edi Vaisbuch, Juan Pedro Kusanovic, Nandor Gabor Than, Tinnakorn Chaiworapongsa, Zhong Dong, Sonia S. Hassan and Roberto Romero* Characterization of visceral and subcutaneous adipose tissue transcriptome in pregnant women with and without spontaneous labor at term: implication of alternative splicing in the metabolic adaptations of adipose tissue to parturition DOI 10.1515/jpm-2015-0259 groups (unpaired analyses) and adipose tissue regions Received July 27, 2015. Accepted October 26, 2015. Previously (paired analyses). Selected genes were tested by quantita- published online March 19, 2016. tive reverse transcription-polymerase chain reaction. Abstract Results: Four hundred and eighty-two genes were differ- entially expressed between visceral and subcutaneous Objective: The aim of this study was to determine gene fat of pregnant women with spontaneous labor at term expression and splicing changes associated with par- (q-value < 0.1; fold change > 1.5). Biological processes turition and regions (visceral vs. subcutaneous) of the enriched in this comparison included tissue and vascu- adipose tissue of pregnant women. lature development as well as inflammatory and meta- Study design: The transcriptome of visceral and abdomi- bolic pathways. Differential splicing was found for 42 nal subcutaneous adipose tissue from pregnant women at genes [q-value < 0.1; differences in Finding Isoforms using term with (n = 15) and without (n = 25) spontaneous labor Robust Multichip Analysis scores > 2] between adipose was profiled with the Affymetrix GeneChip Human Exon tissue regions of women not in labor. Differential exon 1.0 ST array. Overall gene expression changes and the dif- usage associated with parturition was found for three ferential exon usage rate were compared between patient genes (LIMS1, HSPA5, and GSTK1) in subcutaneous tissues. -
Figure S1. Reverse Transcription‑Quantitative PCR Analysis of ETV5 Mrna Expression Levels in Parental and ETV5 Stable Transfectants
Figure S1. Reverse transcription‑quantitative PCR analysis of ETV5 mRNA expression levels in parental and ETV5 stable transfectants. (A) Hec1a and Hec1a‑ETV5 EC cell lines; (B) Ishikawa and Ishikawa‑ETV5 EC cell lines. **P<0.005, unpaired Student's t‑test. EC, endometrial cancer; ETV5, ETS variant transcription factor 5. Figure S2. Survival analysis of sample clusters 1‑4. Kaplan Meier graphs for (A) recurrence‑free and (B) overall survival. Survival curves were constructed using the Kaplan‑Meier method, and differences between sample cluster curves were analyzed by log‑rank test. Figure S3. ROC analysis of hub genes. For each gene, ROC curve (left) and mRNA expression levels (right) in control (n=35) and tumor (n=545) samples from The Cancer Genome Atlas Uterine Corpus Endometrioid Cancer cohort are shown. mRNA levels are expressed as Log2(x+1), where ‘x’ is the RSEM normalized expression value. ROC, receiver operating characteristic. Table SI. Clinicopathological characteristics of the GSE17025 dataset. Characteristic n % Atrophic endometrium 12 (postmenopausal) (Control group) Tumor stage I 91 100 Histology Endometrioid adenocarcinoma 79 86.81 Papillary serous 12 13.19 Histological grade Grade 1 30 32.97 Grade 2 36 39.56 Grade 3 25 27.47 Myometrial invasiona Superficial (<50%) 67 74.44 Deep (>50%) 23 25.56 aMyometrial invasion information was available for 90 of 91 tumor samples. Table SII. Clinicopathological characteristics of The Cancer Genome Atlas Uterine Corpus Endometrioid Cancer dataset. Characteristic n % Solid tissue normal 16 Tumor samples Stagea I 226 68.278 II 19 5.740 III 70 21.148 IV 16 4.834 Histology Endometrioid 271 81.381 Mixed 10 3.003 Serous 52 15.616 Histological grade Grade 1 78 23.423 Grade 2 91 27.327 Grade 3 164 49.249 Molecular subtypeb POLE 17 7.328 MSI 65 28.017 CN Low 90 38.793 CN High 60 25.862 CN, copy number; MSI, microsatellite instability; POLE, DNA polymerase ε. -

Supplementary Table 1
Supplementary Table 1. 492 genes are unique to 0 h post-heat timepoint. The name, p-value, fold change, location and family of each gene are indicated. Genes were filtered for an absolute value log2 ration 1.5 and a significance value of p ≤ 0.05. Symbol p-value Log Gene Name Location Family Ratio ABCA13 1.87E-02 3.292 ATP-binding cassette, sub-family unknown transporter A (ABC1), member 13 ABCB1 1.93E-02 −1.819 ATP-binding cassette, sub-family Plasma transporter B (MDR/TAP), member 1 Membrane ABCC3 2.83E-02 2.016 ATP-binding cassette, sub-family Plasma transporter C (CFTR/MRP), member 3 Membrane ABHD6 7.79E-03 −2.717 abhydrolase domain containing 6 Cytoplasm enzyme ACAT1 4.10E-02 3.009 acetyl-CoA acetyltransferase 1 Cytoplasm enzyme ACBD4 2.66E-03 1.722 acyl-CoA binding domain unknown other containing 4 ACSL5 1.86E-02 −2.876 acyl-CoA synthetase long-chain Cytoplasm enzyme family member 5 ADAM23 3.33E-02 −3.008 ADAM metallopeptidase domain Plasma peptidase 23 Membrane ADAM29 5.58E-03 3.463 ADAM metallopeptidase domain Plasma peptidase 29 Membrane ADAMTS17 2.67E-04 3.051 ADAM metallopeptidase with Extracellular other thrombospondin type 1 motif, 17 Space ADCYAP1R1 1.20E-02 1.848 adenylate cyclase activating Plasma G-protein polypeptide 1 (pituitary) receptor Membrane coupled type I receptor ADH6 (includes 4.02E-02 −1.845 alcohol dehydrogenase 6 (class Cytoplasm enzyme EG:130) V) AHSA2 1.54E-04 −1.6 AHA1, activator of heat shock unknown other 90kDa protein ATPase homolog 2 (yeast) AK5 3.32E-02 1.658 adenylate kinase 5 Cytoplasm kinase AK7 -

Resveratrol Inhibits Cell Cycle Progression by Targeting Aurora Kinase a and Polo-Like Kinase 1 in Breast Cancer Cells
3696 ONCOLOGY REPORTS 35: 3696-3704, 2016 Resveratrol inhibits cell cycle progression by targeting Aurora kinase A and Polo-like kinase 1 in breast cancer cells RUBICELI MEDINA-AGUILAR1, LAURENCE A. Marchat2, ELENA ARECHAGA OCAMPO3, Patricio GARIGLIO1, JAIME GARCÍA MENA1, NICOLÁS VILLEGAS SEPÚlveda4, MACARIO MartÍNEZ CASTILLO4 and CÉSAR LÓPEZ-CAMARILLO5 1Department of Genetics and Molecular Biology, CINVESTAV-IPN, Mexico D.F.; 2Molecular Biomedicine Program and Biotechnology Network, National School of Medicine and Homeopathy, National Polytechnic Institute, Mexico D.F.; 3Natural Sciences Department, Metropolitan Autonomous University, Mexico D.F.; 4Department of Molecular Biomedicine, CINVESTAV-IPN, Mexico D.F.; 5Oncogenomics and Cancer Proteomics Laboratory, Universidad Autónoma de la Ciudad de México, Mexico D.F., Mexico Received December 4, 2015; Accepted January 8, 2016 DOI: 10.3892/or.2016.4728 Abstract. The Aurora protein kinase (AURKA) and the MDA-MB-231 and MCF-7 cells. By western blot assays, we Polo-like kinase-1 (PLK1) activate the cell cycle, and they confirmed that resveratrol suppressed AURKA, CCND1 and are considered promising druggable targets in cancer CCNB1 at 24 and 48 h. In summary, we showed for the first time therapy. However, resistance to chemotherapy and to specific that resveratrol regulates cell cycle progression by targeting small-molecule inhibitors is common in cancer patients; thus AURKA and PLK1. Our findings highlight the potential use of alternative therapeutic approaches are needed to overcome resveratrol as an adjuvant therapy for breast cancer. clinical resistance. Here, we showed that the dietary compound resveratrol suppressed the cell cycle by targeting AURKA Introduction and PLK1 kinases. First, we identified genes modulated by resveratrol using a genome-wide analysis of gene expression Cancer development results from the interaction between in MDA-MB-231 breast cancer cells. -

Molecular Signatures Differentiate Immune States in Type 1 Diabetes Families
Page 1 of 65 Diabetes Molecular signatures differentiate immune states in Type 1 diabetes families Yi-Guang Chen1, Susanne M. Cabrera1, Shuang Jia1, Mary L. Kaldunski1, Joanna Kramer1, Sami Cheong2, Rhonda Geoffrey1, Mark F. Roethle1, Jeffrey E. Woodliff3, Carla J. Greenbaum4, Xujing Wang5, and Martin J. Hessner1 1The Max McGee National Research Center for Juvenile Diabetes, Children's Research Institute of Children's Hospital of Wisconsin, and Department of Pediatrics at the Medical College of Wisconsin Milwaukee, WI 53226, USA. 2The Department of Mathematical Sciences, University of Wisconsin-Milwaukee, Milwaukee, WI 53211, USA. 3Flow Cytometry & Cell Separation Facility, Bindley Bioscience Center, Purdue University, West Lafayette, IN 47907, USA. 4Diabetes Research Program, Benaroya Research Institute, Seattle, WA, 98101, USA. 5Systems Biology Center, the National Heart, Lung, and Blood Institute, the National Institutes of Health, Bethesda, MD 20824, USA. Corresponding author: Martin J. Hessner, Ph.D., The Department of Pediatrics, The Medical College of Wisconsin, Milwaukee, WI 53226, USA Tel: 011-1-414-955-4496; Fax: 011-1-414-955-6663; E-mail: [email protected]. Running title: Innate Inflammation in T1D Families Word count: 3999 Number of Tables: 1 Number of Figures: 7 1 For Peer Review Only Diabetes Publish Ahead of Print, published online April 23, 2014 Diabetes Page 2 of 65 ABSTRACT Mechanisms associated with Type 1 diabetes (T1D) development remain incompletely defined. Employing a sensitive array-based bioassay where patient plasma is used to induce transcriptional responses in healthy leukocytes, we previously reported disease-specific, partially IL-1 dependent, signatures associated with pre and recent onset (RO) T1D relative to unrelated healthy controls (uHC). -

Melatonin Enhances Chondrocytes Survival Through Hyaluronic Acid Production and Mitochondria Protection
Melatonin Enhances Chondrocytes Survival Through Hyaluronic Acid Production and Mitochondria Protection Type Research paper Keywords melatonin, mitochondrion, hyaluronic acid, NF-κB, chondrocyte Abstract Introduction Melatonin, an indoleamine synthesized mainly in the pineal gland, plays a key role in regulating the homeostasis of bone and cartilage. Reactive oxygen species (ROS) are released during osteoarthritis (OA) and associated with cartilage degradation. Melatonin is a potent scavenger of ROS. Material and methods We used rat chondrocytes as a research objective in our study and investigated the effect of melatonin. We evaluated cell viability by MTS, cell apoptosis and mitochondrial transmembrane potential (MMP) by flow cytometry, hyaluronic acid (HA) concentrations by ELISA assay, relative gene and protein expression by RT-qPCR and WB assay, the subcellular location of nuclear factor κB (NF-κB) p65 and Rh-123 dye by immunofluorescence, oxygen consumption rate (OCR) by Seahorse equipment. Results MTS and flow cytometry assay indicated that melatonin prevented H2O2-induced cell death. Melatonin suppressed the expression of HAS1 both from mRNA and protein level in time- and dose- dependent manners. Furthermore, melatonin treatment attenuated the decrease in HA production in response to H2O2 stimulation and repressed the expression of cyclooxygenase-2 (COX-2). Melatonin significantly decreasedPreprint the nuclear volume of p65 and inhibited the expression of p50 induced by H2O2. In addition, melatonin restored the MMP and OCR of -

Transcriptomic Analysis of Early Stages of Intestinal Regeneration in Holothuria Glaberrima David J
Transcriptomic Analysis of Early Stages of Intestinal Regeneration in Holothuria glaberrima David J. Quispe-Parra1, Joshua G. Medina-Feliciano1, Sebastián Cruz-González1, Humberto Ortiz-Zuazaga2, José E. García-Arrarás1* 1University of Puerto Rico, Biology Department, San Juan, 00925, Puerto Rico. 2University of Puerto Rico, Department of Computer Sciences, San Juan, 00925, Puerto Rico. Table S1. Results of transcriptome assessment with BUSCO Parameter BUSCO result Core genes queried 978 Complete core genes detected 99.1% Complete single copy core genes 27.2% Complete duplicated core genes 71.9% Fragmented core genes detected 0.4% Missing core genes 0.5% Table S2. Transcriptome length statistics and composition assessments with gVolante Parameter Result Number of sequences 491 436 Total length (nt) 408 930 895 Longest sequence (nt) 34 610 Shortest sequence (nt) 200 Mean sequence length (nt) 832 N50 sequence length (nt) 1 691 Table S3. RNA-seq data read statistic values Accession Quantity of reads Sample Mapped reads (SRA) Before Filtering After Filtering SRR12564573 NormalA 89 424 588.00 88 112 720.00 94.18% SRR12564572 NormalB 74 105 862.00 72 983 434.00 93.79% SRR12564570 NormalC 60 626 094.00 59 767 896.00 91.55% SRR12564564 Day1A 32 499 910.00 31 721 976.00 90.67% SRR12564563 Day1B 35 164 514.00 34 237 960.00 91.37% SRR12564571 Day1C 43 519 280.00 42 179 220.00 91.53% SRR12564567 Day3A 64 094 536.00 62 995 792.00 90.26% SRR12564566 Day3B 71 654 462.00 70 158 078.00 91.07% SRR12564565 Day3C 71 259 560.00 70 418 332.00 90.75% SRR12564569 Day3D* 107 688 184.00 106 083 898.00 - SRR12564568 Day3E* 108 372 334.00 106 767 522.00 - *Samples used for the assembly but not for differential expression analysis Table S4. -

Multiplexed Surrogate Analysis of Glycotransferase Activity in Whole Biospecimens † † ‡ Chad R
Article pubs.acs.org/ac Multiplexed Surrogate Analysis of Glycotransferase Activity in Whole Biospecimens † † ‡ Chad R. Borges, ,* Douglas S. Rehder, and Paolo Boffetta † Molecular Biomarkers Unit, The Biodesign Institute at Arizona State University, Tempe, Arizona 85287, United States ‡ Institute for Translational Epidemiology and Tisch Cancer Institute, Mount Sinai School of Medicine, New York, New York 10029, United States *S Supporting Information ABSTRACT: Dysregulated glycotransferase enzymes in can- cer cells produce aberrant glycanssome of which can help facilitate metastases. Within a cell, individual glycotransferases promiscuously help to construct dozens of unique glycan structures, making it difficult to comprehensively track their activity in biospecimensespecially where they are absent or inactive. Here, we describe an approach to deconstruct glycans in whole biospecimens then analytically pool together resulting monosaccharide-and-linkage-specific degradation products (“glycan nodes”) that directly represent the activities of specific glycotransferases. To implement this concept, a reproducible, relative quantitation-based glycan methylation analysis methodology was developed that simultaneously captures information from N-, O-, and lipid linked glycans and is compatible with whole biofluids and homogenized tissues; in total, over 30 different glycan nodes are detectable per gas chromatography−mass spectrometry (GC-MS) run. Numerous nonliver organ cancers are known to induce the production of abnormally glycosylated serum proteins. Thus, following analytical validation, in blood plasma, the technique was applied to a group of 59 lung cancer patient plasma samples and age/gender/ smoking-status-matched non-neoplastic controls from the Lung Cancer in Central and Eastern Europe (CEE) study to gauge the clinical utility of the approach toward the detection of lung cancer. -

Figure S1. RNA Degradation Map of the Three Gene Chips. Each of the 76 Samples Is Presented by a Differently Colored Line. Table SI
Figure S1. RNA degradation map of the three gene chips. Each of the 76 samples is presented by a differently colored line. Table SI. Upregulated (n=1,300) and downregulated (n=912) differentially expressed genes. A, Upregulated genes Gene Log fold Average t P-value Adjusted change expression P-value LINGO1 2.6777 6.4170 23.1699 5.54x10-37 1.69x10-33 VWF 1.6093 6.2905 23.0801 7.24x10-37 1.74x10-33 PYCR1 1.4290 7.4945 22.2201 9.80x10-36 1.93x10-32 DTL 2.7697 6.1038 21.9223 2.46x10-35 4.09x10-32 CKAP2L 1.9408 6.7911 21.8106 3.48x10-35 5.31x10-32 CASC5 1.0329 4.9592 21.7923 3.68x10-35 5.31x10-32 RDM1 1.8612 5.8981 21.4909 9.46x10-35 1.28x10-31 HMGA1 1.9524 7.0245 21.2584 1.97x10-34 2.37x10-31 GRHL2 2.3061 6.6285 21.2028 2.35x10-34 2.68x10-31 EMC3-AS1 1.7596 6.4341 20.7583 9.76x10-34 1.06x10-30 DSN1 1.5764 5.6077 20.7126 1.13x10-33 1.16x10-30 CENPF 2.0079 6.1039 20.5981 1.64x10-33 1.48x10-30 BIK 2.8614 6.9592 20.4699 2.48x10-33 2.15x10-30 CDCA8 1.8125 7.2037 20.3946 3.18x10-33 2.45x10-30 CCNB2 1.3002 6.2043 19.9845 1.22x10-32 8.81x10-30 DUSP9 1.7127 6.3549 19.8314 2.03x10-32 1.42x10-29 ESPL1 2.0699 7.4012 19.4946 6.27x10-32 4.11x10-29 CSPG5 2.0283 6.6777 19.3062 1.18x10-31 7.32x10-29 CDCA5 2.5265 7.5517 19.1695 1.88x10-31 1.10x10-28 KIF20B 2.2678 6.4109 19.0425 2.90x10-31 1.57x10-28 E2F1 1.4217 7.0710 19.0116 3.23x10-31 1.70x10-28 CDCA3 2.2727 7.4162 18.9327 4.23x10-31 1.99x10-28 MUC1 2.2947 7.7898 18.8818 5.04x10-31 2.27x10-28 KIF2C 1.6191 7.1943 18.7275 8.57x10-31 3.71x10-28 BIRC5 1.6937 6.5189 18.7158 8.92x10-31 3.78x10-28 CLPB 1.3245 6.0626 -

Global Response of Saccharomyces Cerevisiae to an Alkylating Agent
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 1486–1491, February 1999 Genetics Global response of Saccharomyces cerevisiae to an alkylating agent SCOTT A. JELINSKY AND LEONA D. SAMSON* Department of Cancer Cell Biology, Division of Toxicology, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115 Edited by David Botstein, Stanford University School of Medicine, Stanford, CA, and approved December 7, 1998 (received for review September 16, 1998) ABSTRACT DNA chip technology enables simultaneous CUPr) was used in this study and was grown in 1% yeast examination of how '6,200 Saccharomyces cerevisiae gene tran- extracty2% peptoney2% glucose at 30°C. Cells were grown to a script levels, representing the entire genome, respond to envi- density of 5 3 106 cells per ml as measured by counting duplicated ronmental change. By using chips bearing oligonucleotide ar- dilutions. Cultures were split into two; MMS (0.1%) was added rays, we show that, after exposure to the alkylating agent methyl directly to one culture, and both cultures were incubated at 30°C ' methanesulfonate, 325 gene transcript levels are increased and for 1 h. Cells were pelleted and washed once in distilled H2O and '76 are decreased. Of the 21 genes that already were known to once in AE buffer (50 mM NaOAc, pH 5.2y10 mM EDTA) be induced by a DNA-damaging agent, 18 can be scored as immediately before RNA extraction. inducible in this data set, and surprisingly, most of the newly RNA Extraction. Total RNA was isolated by using a hot-phenol identified inducible genes are induced even more strongly than method (15).