Characterization of Visceral and Subcutaneous Adipose Tissue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
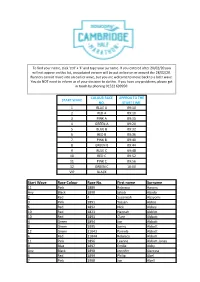
Start Wave Race Colour Race No. First Name Surname
To find your name, click 'ctrl' + 'F' and type your surname. If you entered after 20/02/20 you will not appear on this list, an updated version will be put online on or around the 28/02/20. Runners cannot move into an earlier wave, but you are welcome to move back to a later wave. You do NOT need to inform us of your decision to do this. If you have any problems, please get in touch by phoning 01522 699950. COLOUR RACE APPROX TO THE START WAVE NO. START TIME 1 BLUE A 09:10 2 RED A 09:10 3 PINK A 09:15 4 GREEN A 09:20 5 BLUE B 09:32 6 RED B 09:36 7 PINK B 09:40 8 GREEN B 09:44 9 BLUE C 09:48 10 RED C 09:52 11 PINK C 09:56 12 GREEN C 10:00 VIP BLACK Start Wave Race Colour Race No. First name Surname 11 Pink 1889 Rebecca Aarons Any Black 1890 Jakob Abada 2 Red 4 Susannah Abayomi 3 Pink 1891 Yassen Abbas 6 Red 1892 Nick Abbey 10 Red 1823 Hannah Abblitt 10 Red 1893 Clare Abbott 4 Green 1894 Jon Abbott 8 Green 1895 Jonny Abbott 12 Green 11043 Pamela Abbott 6 Red 11044 Rebecca Abbott 11 Pink 1896 Leanne Abbott-Jones 9 Blue 1897 Emilie Abby Any Black 1898 Jennifer Abecina 6 Red 1899 Philip Abel 7 Pink 1900 Jon Abell 10 Red 600 Kirsty Aberdein 6 Red 11045 Andrew Abery Any Black 1901 Erwann ABIVEN 11 Pink 1902 marie joan ablat 8 Green 1903 Teresa Ablewhite 9 Blue 1904 Ahid Abood 6 Red 1905 Alvin Abraham 9 Blue 1906 Deborah Abraham 6 Red 1907 Sophie Abraham 1 Blue 11046 Mitchell Abrams 4 Green 1908 David Abreu 11 Pink 11047 Kathleen Abuda 10 Red 11048 Annalisa Accascina 4 Green 1909 Luis Acedo 10 Red 11049 Vikas Acharya 11 Pink 11050 Catriona Ackermann -
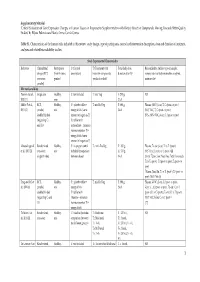
Critical Evaluation of Gene Expression Changes in Human Tissues In
Supplementary Material ‘Critical Evaluation of Gene Expression Changes in Human Tissues in Response to Supplementation with Dietary Bioactive Compounds: Moving Towards Better-Quality Studies’ by Biljana Pokimica and María-Teresa García-Conesa Table S1. Characteristics of the human trials included in this review: study design, type of participants, control and intervention description, dose and duration of treatment, analyses and related bioavailability studies. Study Experimental Characteristics Reference Clinical trial Participants C (Control T (Treatment with Total daily dose, Bioavailability studies: type of sample, design (RCT, (health status, description) bioactive compounds, duration (d or h)1 compounds and (or) metabolites analysed, crossover, gender) products or diet) main results2 parallel) Mix meals and diets Persson I et al., Single arm Healthy, C: not included T: mix Veg T: 250 g, NR 2000 [1] men 21 d Møller P et al., RCT, Healthy, C1: placebo tablet + T: mix FruVeg T: 600 g, Plasma: (NS↑) β-car, T, C2 (post- vs pre-) 2003 [2] parallel, mix energy drink (same 24 d (NC) VitC, T, C2 (post- vs pre-) double blinded amount of sugars as T) (NS↓, 69%) VitC, β-car, C1 (post- vs pre-) (regarding C1 C2: tablet with and C2) antioxidants + minerals (same amount as T) + energy drink (same amount of sugars as T) Almendingen K Randomized, Healthy, C: no proper control T1,2: mix FruVeg T1: 300 g, Plasma: ↑α-car, β-car, T2 vs T1 (post-) et al., 2005 [3] crossover, mix included (comparison T2: 750 g, (NS↑) Lyc, Lut, T2 vs T1 (post-) [4] single -

IN TAX LEADERS WOMEN in TAX LEADERS | 4 AMERICAS Latin America
WOMEN IN TAX LEADERS THECOMPREHENSIVEGUIDE TO THE WORLD’S LEADING FEMALE TAX ADVISERS SIXTH EDITION IN ASSOCIATION WITH PUBLISHED BY WWW.INTERNATIONALTAXREVIEW.COM Contents 2 Introduction and methodology 8 Bouverie Street, London EC4Y 8AX, UK AMERICAS Tel: +44 20 7779 8308 4 Latin America: 30 Costa Rica Fax: +44 20 7779 8500 regional interview 30 Curaçao 8 United States: 30 Guatemala Editor, World Tax and World TP regional interview 30 Honduras Jonathan Moore 19 Argentina 31 Mexico Researchers 20 Brazil 31 Panama Lovy Mazodila 24 Canada 31 Peru Annabelle Thorpe 29 Chile 32 United States Jason Howard 30 Colombia 41 Venezuela Production editor ASIA-PACIFIC João Fernandes 43 Asia-Pacific: regional 58 Malaysia interview 59 New Zealand Business development team 52 Australia 60 Philippines Margaret Varela-Christie 53 Cambodia 61 Singapore Raquel Ipo 54 China 61 South Korea Managing director, LMG Research 55 Hong Kong SAR 62 Taiwan Tom St. Denis 56 India 62 Thailand 58 Indonesia 62 Vietnam © Euromoney Trading Limited, 2020. The copyright of all 58 Japan editorial matter appearing in this Review is reserved by the publisher. EUROPE, MIDDLE EAST & AFRICA 64 Africa: regional 101 Lithuania No matter contained herein may be reproduced, duplicated interview 101 Luxembourg or copied by any means without the prior consent of the 68 Central Europe: 102 Malta: Q&A holder of the copyright, requests for which should be regional interview 105 Malta addressed to the publisher. Although Euromoney Trading 72 Northern & 107 Netherlands Limited has made every effort to ensure the accuracy of this Southern Europe: 110 Norway publication, neither it nor any contributor can accept any regional interview 111 Poland legal responsibility whatsoever for consequences that may 86 Austria 112 Portugal arise from errors or omissions, or any opinions or advice 87 Belgium 115 Qatar given. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Wayne County Death Index, 1934-1939 1 SURNAME FIRST
SURNAME FIRST NAME MIDDLE AGE DATE LOCATION BOX FILE Aaron Phillip W 305 4/21/1938 WD 21 16 Aarons Victoria 149 10/9/1937 EC 13 16 Abbeg Minnie Manning 160 7/13/1938 SM TP 21 8 Abbey Thomas J 180 8/7/1939 HP 30 16 Abbote Vito 165 3/3/1938 NK TP 23 7 Abbott Ada 180 3/2/1938 HP 22 3 Abbott Blanche Maude 152 5/24/1938 HP 22 7 Abbott Chester 155 10/21/1938 WD 21 20 Abbott Ira W 190 8/20/1935 WY 6 11 Abbott Melvin J 102 3/31/1936 WD 9 15 Abbott Roy 142 6/24/1937 WD 15 19 Abbott William C 184 9/28/1936 RM TP 8 14 Abell Ellen 163 2/20/1937 NK TP 17 8 Abelson James H 183 11/27/1938 NK TP 24 12 Aben Augusta 171 11/25/1937 LP 14 15 Abernathy Paul 152 4/3/1939 NK TP 28 16 Abjorson Sven J 148 8/2/1938 GPF 19 16 Abraham Anna 155 4/3/1938 HP 22 5 Abraham Fay 142 9/17/1939 WD 27 22 Abraham Frank Wilson 119 2/18/1937 HP 16 4 Abraham Omar 150 8/2/1938 NK TP 24 1 Abrahamson John 157 12/22/1937 NK TP 18 16 Abram William 175 11/3/1937 NK TP 18 12 Abshagen William 163 2/11/1937 NK TP 17 7 Acciaccafero Dora 159 5/21/1939 NK TP 28 23 Acker Baby 0 4/12/1936 ML 7 27 Acker William H 187 8/12/1939 HP 30 16 Ackerman Jessie W 165 5/31/1939 WD 27 18 Ackerman Leo 174 4/15/1935 NK TP 4 14 Ackermann Joseph M 167 4/15/1936 HP 10 7 Ackersville William 141 10/30/1935 HP 3 19 Ackley Robert D 106 6/28/1936 HP 10 12 Adach John 150 8/17/1939 NK TP 29 7 Adair Baby 0 8/28/1939 WD 27 21 Adair Kristine Ann 105 5/6/1937 WD 15 17 Adamczak Baby 0 7/8/1935 HM 1 29 Adamczak Veronika 301 1/14/1939 HM 31 1 Adamczyk Baby 0 8/3/1937 HM 14 8 Adamczyk Donald 204 2/6/1937 HM 14 2 Adamczyk -

Family Group Sheets Surname Index
PASSAIC COUNTY HISTORICAL SOCIETY FAMILY GROUP SHEETS SURNAME INDEX This collection of 660 folders contains over 50,000 family group sheets of families that resided in Passaic and Bergen Counties. These sheets were prepared by volunteers using the Societies various collections of church, ceme tery and bible records as well as city directo ries, county history books, newspaper abstracts and the Mattie Bowman manuscript collection. Example of a typical Family Group Sheet from the collection. PASSAIC COUNTY HISTORICAL SOCIETY FAMILY GROUP SHEETS — SURNAME INDEX A Aldous Anderson Arndt Aartse Aldrich Anderton Arnot Abbott Alenson Andolina Aronsohn Abeel Alesbrook Andreasen Arquhart Abel Alesso Andrews Arrayo Aber Alexander Andriesse (see Anderson) Arrowsmith Abers Alexandra Andruss Arthur Abildgaard Alfano Angell Arthurs Abraham Alje (see Alyea) Anger Aruesman Abrams Aljea (see Alyea) Angland Asbell Abrash Alji (see Alyea) Angle Ash Ack Allabough Anglehart Ashbee Acker Allee Anglin Ashbey Ackerman Allen Angotti Ashe Ackerson Allenan Angus Ashfield Ackert Aller Annan Ashley Acton Allerman Anners Ashman Adair Allibone Anness Ashton Adams Alliegro Annin Ashworth Adamson Allington Anson Asper Adcroft Alliot Anthony Aspinwall Addy Allison Anton Astin Adelman Allman Antoniou Astley Adolf Allmen Apel Astwood Adrian Allyton Appel Atchison Aesben Almgren Apple Ateroft Agar Almond Applebee Atha Ager Alois Applegate Atherly Agnew Alpart Appleton Atherson Ahnert Alper Apsley Atherton Aiken Alsheimer Arbuthnot Atkins Aikman Alterman Archbold Atkinson Aimone -

Surname Folders.Pdf
SURNAME Where Filed Aaron Filed under "A" Misc folder Andrick Abdon Filed under "A" Misc folder Angeny Abel Anger Filed under "A" Misc folder Aberts Angst Filed under "A" Misc folder Abram Angstadt Achey Ankrum Filed under "A" Misc folder Acker Anns Ackerman Annveg Filed under “A” Misc folder Adair Ansel Adam Anspach Adams Anthony Addleman Appenzeller Ader Filed under "A" Misc folder Apple/Appel Adkins Filed under "A" Misc folder Applebach Aduddell Filed under “A” Misc folder Appleman Aeder Appler Ainsworth Apps/Upps Filed under "A" Misc folder Aitken Filed under "A" Misc folder Apt Filed under "A" Misc folder Akers Filed under "A" Misc folder Arbogast Filed under "A" Misc folder Albaugh Filed under "A" Misc folder Archer Filed under "A" Misc folder Alberson Filed under “A” Misc folder Arment Albert Armentrout Albight/Albrecht Armistead Alcorn Armitradinge Alden Filed under "A" Misc folder Armour Alderfer Armstrong Alexander Arndt Alger Arnold Allebach Arnsberger Filed under "A" Misc folder Alleman Arrel Filed under "A" Misc folder Allen Arritt/Erret Filed under “A” Misc folder Allender Filed under "A" Misc folder Aschliman/Ashelman Allgyer Ash Filed under “A” Misc folder Allison Ashenfelter Filed under "A" Misc folder Allumbaugh Filed under "A" Misc folder Ashoff Alspach Asper Filed under "A" Misc folder Alstadt Aspinwall Filed under "A" Misc folder Alt Aston Filed under "A" Misc folder Alter Atiyeh Filed under "A" Misc folder Althaus Atkins Filed under "A" Misc folder Altland Atkinson Alwine Atticks Amalong Atwell Amborn Filed under -

Localized Holes and Delocalized Electrons in Photoexcited Inorganic Perovskites: Watching Each Atomic Actor by Picosecond X-Ray Absorption Spectroscopy Fabio G
Localized holes and delocalized electrons in photoexcited inorganic perovskites: Watching each atomic actor by picosecond X-ray absorption spectroscopy Fabio G. Santomauro, Jakob Grilj, Lars Mewes, Georgian Nedelcu, Sergii Yakunin, Thomas Rossi, Gloria Capano, André Al Haddad, James Budarz, Dominik Kinschel, Dario S. Ferreira, Giacomo Rossi, Mario Gutierrez Tovar, Daniel Grolimund, Valerie Samson, Maarten Nachtegaal, Grigory Smolentsev, Maksym V. Kovalenko, and Majed Chergui Citation: Structural Dynamics 4, 044002 (2017); doi: 10.1063/1.4971999 View online: http://dx.doi.org/10.1063/1.4971999 View Table of Contents: http://aca.scitation.org/toc/sdy/4/4 Published by the American Institute of Physics Articles you may be interested in Structural enzymology using X-ray free electron lasers Structural Dynamics 4, 044003 (2016); 10.1063/1.4972069 A comparison of the innate flexibilities of six chains in F1-ATPase with identical secondary and tertiary folds; 3 active enzymes and 3 structural proteins Structural Dynamics 4, 044001 (2016); 10.1063/1.4967226 The image of scientists in The Big Bang Theory Physics Today 70, 40 (2017); 10.1063/PT.3.3427 Ultrafast electron crystallography of the cooperative reaction path in vanadium dioxide Structural Dynamics 3, 034304 (2016); 10.1063/1.4953370 A general method for baseline-removal in ultrafast electron powder diffraction data using the dual-tree complex wavelet transform Structural Dynamics 4, 044004 (2016); 10.1063/1.4972518 A beam branching method for timing and spectral characterization of hard X-ray free-electron lasers Structural Dynamics 3, 034301 (2016); 10.1063/1.4939655 STRUCTURAL DYNAMICS 4, 044002 (2017) Localized holes and delocalized electrons in photoexcited inorganic perovskites: Watching each atomic actor by picosecond X-ray absorption spectroscopy Fabio G. -

GSTK1 Rabbit Pab
Leader in Biomolecular Solutions for Life Science GSTK1 Rabbit pAb Catalog No.: A5226 1 Publications Basic Information Background Catalog No. This gene encodes a member of the kappa class of the glutathione transferase A5226 superfamily of enzymes that function in cellular detoxification. The encoded protein is localized to the peroxisome and catalyzes the conjugation of glutathione to a wide Observed MW range of hydrophobic substates facilitating the removal of these compounds from cells. 25KDa Alternative splicing results in multiple transcript variants. Calculated MW 20kDa/24kDa/25kDa/31kDa Category Primary antibody Applications WB, IHC, IF Cross-Reactivity Human, Mouse, Rat Recommended Dilutions Immunogen Information WB 1:500 - 1:2000 Gene ID Swiss Prot 373156 Q9Y2Q3 IHC 1:50 - 1:100 Immunogen 1:50 - 1:100 IF Recombinant fusion protein containing a sequence corresponding to amino acids 1-226 of human GSTK1 (NP_057001.1). Synonyms GSTK1;GST;GST 13-13;GST13;GST13-13;GSTK1-1;hGSTK1 Contact Product Information www.abclonal.com Source Isotype Purification Rabbit IgG Affinity purification Storage Store at -20℃. Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide,50% glycerol,pH7.3. Validation Data Western blot analysis of extracts of various cell lines, using GSTK1 antibody (A5226) at 1:1000 dilution. Secondary antibody: HRP Goat Anti-Rabbit IgG (H+L) (AS014) at 1:10000 dilution. Lysates/proteins: 25ug per lane. Blocking buffer: 3% nonfat dry milk in TBST. Detection: ECL Basic Kit (RM00020). Exposure time: 60s. Immunohistochemistry of paraffin- Immunohistochemistry of paraffin- Immunohistochemistry of paraffin- embedded human lung cancer using embedded human liver cancer using embedded human thyroid cancer using GSTK1 Rabbit pAb (A5226) at dilution of GSTK1 Rabbit pAb (A5226) at dilution of GSTK1 Rabbit pAb (A5226) at dilution of 1:25 (40x lens). -
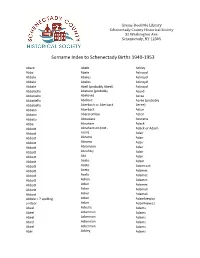
Surname Index to Schenectady Births 1940-1953
Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Abare Abele Ackley Abba Abele Ackroyd Abbale Abeles Ackroyd Abbale Abeles Ackroyd Abbale Abell (probably Abeel) Ackroyd Abbatiello Abelone (probably Acord Abbatiello Abelove) Acree Abbatiello Abelove Acree (probably Abbatiello Aberbach or Aberback Aeree) Abbato Aberback Acton Abbato Abercrombie Acton Abbato Aboudara Acucena Abbe Abraham Adack Abbott Abrahamson (not - Adack or Adach Abbott nson) Adair Abbott Abrams Adair Abbott Abrams Adair Abbott Abramson Adair Abbott Abrofsky Adair Abbott Abt Adair Abbott Aceto Adam Abbott Aceto Adamczak Abbott Aceto Adamec Abbott Aceto Adamec Abbott Acken Adamec Abbott Acker Adamec Abbott Acker Adamek Abbott Acker Adamek Abbzle = ? spelling Acker Adamkiewicz unclear Acker Adamkiewicz Abeel Ackerle Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abel Ackley Adams Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Adams Adamson Ahl Adams Adanti Ahles Adams Addis Ahman Adams Ademec or Adamec Ahnert Adams Adinolfi Ahren Adams Adinolfi Ahren Adams Adinolfi Ahrendtsen Adams Adinolfi Ahrendtsen Adams Adkins Ahrens Adams Adkins Ahrens Adams Adriance Ahrens Adams Adsit Aiken Adams Aeree Aiken Adams Aernecke Ailes = ? Adams Agans Ainsworth Adams Agans Aker (or Aeher = ?) Adams Aganz (Agans ?) Akers Adams Agare or Abare = ? Akerson Adams Agat Akin Adams Agat Akins Adams Agen Akins Adams Aggen Akland Adams Aggen Albanese Adams Aggen Alberding Adams Aggen Albert Adams Agnew Albert Adams Agnew Albert or Alberti Adams Agnew Alberti Adams Agostara Alberti Adams Agostara (not Agostra) Alberts Adamski Agree Albig Adamski Ahave ? = totally Albig Adamson unclear Albohm Adamson Ahern Albohm Adamson Ahl Albohm (not Albolm) Adamson Ahl Albrezzi Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. -
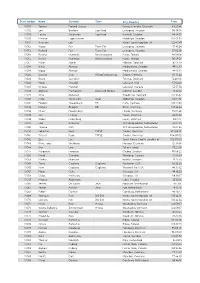
Start Number Name Surname Team City, Country Time 11001 Tommy
Start number Name Surname Team City, Country Time 11001 Tommy Frølund Jensen Kongens lyngby, Denmark 89:20:46 11002 Emil Boström Lag Nord Linköping, Sweden 98:19:04 11003 Lovisa Johansson Lag Nord Burträsk, Sweden 98:19:02 11005 Maurice Lagershausen Huddinge, Sweden 102:01:54 11011 Hyun jun Lee Korea, (south) republic of 122:40:49 11012 Viggo Fält Team Fält Linköping, Sweden 57:45:24 11013 Richard Fält Team Fält Linköping, Sweden 57:45:28 11014 Rasmus Nummela Rackmannarna Pjelax, Finland 98:59:06 11015 Rafael Nummela Rackmannarna Pjelax, Finland 98:59:06 11017 Marie Zølner Hillerød, Denmark 82:11:49 11018 Niklas Åkesson Mellbystrand, Sweden 99:45:22 11019 Viggo Åkesson Mellbystrand, Sweden 99:45:22 11020 Damian Sulik #StopComplaining Siegen, Germany 101:13:24 11023 Bruno Svendsen Tylstrup, Denmark 74:48:08 11024 Philip Oswald Lakeland, USA 55:52:02 11025 Virginia Marshall Lakefield, Canada 55:57:54 11026 Andreas Mathiasson Backyard Heroes Ödsmål, Sweden 75:12:02 11028 Stine Andersen Brædstrup, Denmark 104:35:37 11030 Patte Johansson Tiger Södertälje, Sweden 80:24:51 11031 Frederic Wiesenbach BB Dahn, Germany 101:21:50 11032 Romea Brugger BB Berlin, Germany 101:22:44 11034 Oliver Freudenberg Heyda, Germany 75:07:48 11035 Jan Hennig Farum, Denmark 82:11:50 11036 Robert Falkenberg Farum, Denmark 82:11:51 11037 Edo Boorsma Sint annaparochie, Netherlands 123:17:34 11038 Trijneke Stuit Sint annaparochie, Netherlands 123:17:35 11040 Sebastian Keck TATSE Gieäen, Germany 104:08:01 11041 Tatjana Kage TATSE Gieäen, Germany 104:08:02 11042 Eun Lee -

Whole Egg Consumption Increases Gene Expression Within the Glutathione Pathway in the Liver of Zucker Diabetic Fatty Rats
Food Science and Human Nutrition Publications Food Science and Human Nutrition 11-3-2020 Whole egg consumption increases gene expression within the glutathione pathway in the liver of Zucker Diabetic Fatty rats Joe L. Webb Iowa State University Amanda E. Bries Iowa State University, [email protected] Brooke Vogel Iowa State University Claudia Carrillo Iowa State University, [email protected] Lily Harvison Iowa State University, [email protected] See next page for additional authors Follow this and additional works at: https://lib.dr.iastate.edu/fshn_hs_pubs Part of the Dietetics and Clinical Nutrition Commons, Endocrinology, Diabetes, and Metabolism Commons, Exercise Science Commons, Food Chemistry Commons, Human and Clinical Nutrition Commons, and the Molecular, Genetic, and Biochemical Nutrition Commons The complete bibliographic information for this item can be found at https://lib.dr.iastate.edu/ fshn_hs_pubs/38. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Food Science and Human Nutrition at Iowa State University Digital Repository. It has been accepted for inclusion in Food Science and Human Nutrition Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Whole egg consumption increases gene expression within the glutathione pathway in the liver of Zucker Diabetic Fatty rats Abstract Nutrigenomic evidence supports the idea that Type 2 Diabetes Mellitus (T2DM) arises due to the interactions between the transcriptome, individual genetic profiles, lifestyle, and diet. Since eggs are a nutrient dense food containing bioactive ingredients that modify gene expression, our goal was to examine the role of whole egg consumption on the transcriptome during T2DM.