Nelson Et Al No Highlight-1 Edit Changes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evidence of Lingual-Luring by an Aquatic Snake
Journal of Herpetology, Vol. 34 No. 1 pp 67-74, 2000 Copyright 2000 Society for the Study of Amphibians and Reptiles Evidence of Lingual-luring by an Aquatic Snake HARTWELL H. WELSH, JR. AND AMY J. LIND Pacific Southwest Research Station, USDA Forest Service, 1700 Bayview Dr., Arcata, California 95521, USA. E-mail: hwelsh/[email protected] ABSTRACT.-We describe and quantify the components of an unusual snake behavior used to attract fish prey: lingual-luring. Our earlier research on the foraging behavior of the Pacific Coast aquatic garter snake (Thamnophis atratus) indicated that adults are active foragers, feeding primarily on aquatic Pacific giant salamanders (Dicamptodon tenebrosus) in streambed substrates. Juvenile snakes, however, use primarily ambush tactics to capture larval anurans and juvenile salmonids along stream margins, behaviors that include the lingual-luring described here. We found that lingual-luring differed from typical chemosensory tongue-flicking by the position of the snake, contact of the tongue with the water surface, and the length of time the tongue was extended. Luring snakes are in ambush position and extend and hold their tongues out rigid, with the tongue-tips quivering on the water surface, apparently mimicking insects in order to draw young fish within striking range. This behavior is a novel adaptation of the tongue-vomeronasal system by a visually-oriented predator. The luring of prey by snakes has been asso- luring function (Mushinsky, 1987; Ford and ciated primarily with the use of the tail, a be- Burghardt, 1993). However, Lillywhite and Hen- havior termed caudal luring (e.g., Neill, 1960; derson (1993) noted the occurrence of a pro- Greene and Campbell, 1972; Heatwole and Dav- longed extension of the tongue observed in vine ison, 1976; Jackson and Martin, 1980; Schuett et snakes (e.g., Kennedy, 1965; Henderson and al., 1984; Chizar et al., 1990). -

This Article Appeared in a Journal Published by Elsevier. the Attached Copy Is Furnished to the Author for Internal Non-Commerci
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/authorsrights Author's personal copy Molecular Phylogenetics and Evolution 71 (2014) 149–156 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A multi-locus molecular phylogeny for Australia’s iconic Jacky Dragon (Agamidae: Amphibolurus muricatus): Phylogeographic structure along the Great Dividing Range of south-eastern Australia ⇑ Mitzy Pepper a, , Marco D. Barquero b, Martin J. Whiting b, J. Scott Keogh a a Division of Evolution, Ecology and Genetics, Research School of Biology, The Australian National University, Canberra, Australia b Department of Biological Sciences, Macquarie University, Sydney, Australia article info abstract Article history: Jacky dragons (Amphibolurus muricatus) are ubiquitous in south-eastern Australia and were one of the Received 25 June 2013 -

Reproductive Ecology of the Mountain Dragon, Rankin/A (Tympanocryptis) Diemensis (Reptilia: Squamata: Agamidae) in Tasmania
Papers and Proceedings ofthe Royal Society of Tasmania, Volume 139, 2005 23 REPRODUCTIVE ECOLOGY OF THE MOUNTAIN DRAGON, RANKIN/A (TYMPANOCRYPTIS) DIEMENSIS (REPTILIA: SQUAMATA: AGAMIDAE) IN TASMANIA by Jemina Stuart-Smith, Roy Swain and Andrew Welling (with three tables and two text-figures) Stuart-Smith, J., Swain, R. & Welling, A. 2005 (16:xii): Reproductive ecology of the Mountain Dragon, Rankinia (Tympanocryptis) diemensis (Reptilia: Squamata: Agamidae) in Tasmania. Papers and Proceedings of the Royal Society of Tasmania 139: 23-28. https://doi.org/10.26749/rstpp.139.23 ISSN 0080-4703. School of Zoology, University of Tasmania, Private Bag 5, Tasmania, 7001 Australia.(J.S-S.*, R.S., AW). *Author for correspondence. The mountain dragon, Rankinia (Tympanocryptis) diemensis (Gray, 1841), is the only member of the Agamidae in Tasmania. It occurs in some of the coldest regions occupied by any dragon in Australia, and is found in a variety of habitats ranging from coastal heath to alpine scrub. This paper examines the reproductive ecology of R. diemensis in the most southerly range of its distribution, providing baseline data on timing of reproductive events, reproductive cycles, nesting behaviour and ovipositioning, clutch characteristics and incubation conditions. Winter torpor lasts approximately seven months with males emerging in early September and spermatogenesis occurring from September-November. Females emerge later, with vitcllogenesis occurring from September-December. Gravid females may be found between October and January, but females are non-vitcllogenic from late December until the following season. The firstclutch is typically laid from October-December, with a variable clutch size (2-11 eggs). Females store sperm and a second clutch may be laid fiveweeks after the first. -

Caudal Distraction by Rat Snakes (Colubridae, Elaphe): a Novel Behavior Used When Capturing Mammalian Prey
Great Basin Naturalist Volume 59 Number 4 Article 8 10-15-1999 Caudal distraction by rat snakes (Colubridae, Elaphe): a novel behavior used when capturing mammalian prey Stephen J. Mullin University of Memphis, Memphis, Tennessee Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Mullin, Stephen J. (1999) "Caudal distraction by rat snakes (Colubridae, Elaphe): a novel behavior used when capturing mammalian prey," Great Basin Naturalist: Vol. 59 : No. 4 , Article 8. Available at: https://scholarsarchive.byu.edu/gbn/vol59/iss4/8 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Great Ba....in Naturalist 59(4), ©1999, pp. 361....167 CAUDAL DISTRACTION BY RAT SNAKES (COLUBHIDAE, ELAPHE): A NOVEL BEHAVIOR USED WHEN CAPTURING MAMMALIAN PREY Stephen]. Mullin1 AJ3S11UCT.--el.mthtl movement in snakes trulY serve ei.ther a pl'Cdatory (e.g., caudal luring) or defensive (e.g., rattling, aposem,ttism) fUllction, I descliho n new behavioral pattern of tai.l movement in snakes. Gray rat snakl.'$ (Elaphe OhSO!etd spiloid.es) fi)raging on ~ma11 mmnmnls (Mus d01ne~·ticus) Inoved. their tails in un erratic, whiplike fashion uIter detecting prey in their vidnity. The thrashing movement in the horizontal plfme was audibly and visually obviolls, resulting in dis placement of leaf litter around the hlil. All subjects displayed the behavior, hilt not in all foraging episodes. -

Spring 2009 RURAL LIVING in ARIZONA Volume 3, Number 2
ARIZONA COOPERATIVE E TENSION THE UNIVERSITY OF ARIZONA COLLEGE OF AGRICULTURE AND LIFE SCIENCES Backyards& Beyond Spring 2009 RURAL LIVING IN ARIZONA Volume 3, Number 2 Spring 2009 1 Common Name: Globemallow Scientific Name: Sphaeralcea spp. Globemallow is a common native wildflower found throughout most of Arizona. There are 16 species (and several varieties) occurring in the state, the majority of which are perennials. They are found between 1,000 and 6,000 feet in elevation and grow on a variety of soil types. Depending on the species, globemallows are either herbaceous or slightly woody at the base of the plant and grow between 2-3 feet in height (annual species may only grow to 6 inches). The leaves are three-lobed, and while the shape varies by species, they are similar enough to help identify the plant as a globemallow. The leaves have star-shaped hairs that give the foliage a gray-green color. Flower color Plant Susan Pater varies from apricot (the most common) to red, pink, lavender, pale yellow and white. Many of the globemallows flower in spring and again in summer. Another common name for globemallow is sore-eye poppy (mal de ojos in Spanish), from claims that the plant irritates the eyes. In southern California globemallows are known as plantas muy malas, translated to mean very bad plants. Ironically, the Pima Indian name for globemallow means a cure for sore eyes. The Hopi Indians used the plant for healing certain ailments and the stems as a type of chewing gum, and call the plant kopona. -

2007 Australasian Society for the Study of Animal Behaviour & the International Union for the Study of Social Insects (Australian Chapter)
ASSAB 2007 AUSTRALASIAN SOCIETY FOR THE STUDY OF ANIMAL BEHAVIOUR & THE INTERNATIONAL UNION FOR THE STUDY OF SOCIAL INSECTS (AUSTRALIAN CHAPTER) 12-15 April 2007 The Australian National University Canberra Venue Robertson Lecture Theatre Research School of Biological Sciences Building 46E Hosted by the Research School of Biological Sciences 2 Sponsored by the ARC Centre of Excellence in Vision Science (ACEVS) http://www.vision.edu.au/ LOCAL HOSTS: JOCHEN ZEIL Visual Sciences, Research School of Biological Sciences The Australian National University AJAY NARENDRA Visual Sciences, Research School of Biological Sciences The Australian National University ROB HEINSOHN Centre for Resource and Environmental Studies The Australian National University JAN HEMMI Visual Sciences, Research School of Biological Sciences The Australian National University RICHARD PETERS Visual Sciences, Research School of Biological Sciences The Australian National University WITH SPECIAL THANKS TO THE ASSAB TREASURER, XIMENA NELSON & THE ASSAB PRESIDENT PHIL TAYLOR FOR THEIR SUPPORT Thursday, 12 April Friday, 13 April Saturday, 14 April Sunday, 15 April 8:00 Plenary Lecture Tinbergen Centenary Lecture IUSSI Lecture 9:00 ASSAB 2007 Barbara Webb Chris Evans & Jochen Zeil Ryszard Maleszka 12 - 15 April 2007 09:30 09:30 09:30 Session 3: RSBS Session 8: Session 12: 10:00 SENSORY SYSTEMS & SOCIAL INSECTS I COMMUNICATION I HOMING & NAVIGATION Tea/CoffeeTea/Coffee Break Break Tea/Coffee Break 11:00 Session 9: FORAGING, COMPETITION & Session 4: LIFE HISTORIES I Session 13: -

Australian Capital Territory & Yass Valley Reptiles
Australian Capital Territory & Yass Valley Reptiles Geoff Robertson 4 June 2017 Turtle (1-2 species) Lizard Gecko (2) Legless lizard (4-5) Dragon (6) Gonna (2) Skink (32-35) Egernia group - chunky (9) Eugongylus group - striped (9-10) Sphenomorphus group - red (14) Snakes Blind (1) Total species = 57-66 Python (1?) Elapid (9-12) Murray (short-neck) turtle (above left) Emydura macquarii Eastern long-neck turtle (above right) Chelodina longicollis Lizards Monitors (goannas) Dragons Skinks Geckos Measuring length lizards & snakes: Legless TL - total length. lizards SVL - tip of the nose (snout) to vent. Dragons Physignathus lesueurii, Australia: 11 genera, 70 species. SVL 245 mm ACT/Yass: 5 genera, 6 species Common: Pogona barbata, Water dragon (top), Aust: 1 SVL 250 mm species. Eastern bearded dragon (bottom), Aust: 6 species. Jacky lizard or tree dragon. Three similar dragons Jacky (top), Nobbi (middle) and Mountain (bottom). SVL is Jacky - 120mm Nobbi - 84mm Mountain - 82mm However, all dragons (including water & bearded ) look similar when young. Photos top to bottom - Amphibolurus mutinous and A. nobbi, & Rankina diemensis. by Warren Saunders, John Wombey and Roger Farrow. Grassland earless dragon Eight earless dragon species in Australia. GED found in some natural grassland sites, climbs tussock grasses otherwise a ground dweller. Unlike other dragons, Tail slight/curls. Populations - ACT & southern Monaro. GED thought extinct, re-discovered, crashed in drought, captive breeding, future? Tympanocryptis pinguicolla. SVL 55mm. Bottom photo: Margaret Ning. Jacky dragon Bearded dragon Water dragon Nobbi dragon Mountain dragon Grassland earless dragon Gecko Australia: 18 genera & 111 species ACT/Yass: 2 species Stone gecko (right) Marble gecko (below) Diplodactylus vittatus, SVL 50mm Photo John Wombey Christinus marmaratus, SVL 70mm Photo Margaret Ning Legless lizards Related to geckos Australia: 7 genera & 38 species ACT/Yass: 2 genera & 5 species Unlike snakes: Lialis burtonis, SVL170mm Photo John Wombey • Large fleshy tongue (cf forked). -

Wollongong Campus Fauna Species List
FACTSHEET Wollongong Campus Fauna Species List The Wollongong Campus landscape provides habitat for a diverse range of animals. Animals that are known or potentially occur on campus are listed below. Key: # Introduced species, * Listed as a Vulnerable or threatened species in NSW, C- common, E - exotic, Res- resident, Br - breeds at UOW, M - migratory, V – visitor, P – potentially occurs onsite AMPHIBIANS Bleating tree frog Litoria dentate Res Eastern dwarf tree frog Litoria fallax Res Eastern froglet Crinia signifera C, Res Leaf green tree frog Litoria nudidigita Res Peron’s tree frog Litoria peronei Res Striped marsh frog Limnodynastes peronei Res Tyler’s tree frog Litoria tyleri Res Verreaux’s tree frog Litoria verreauxi Res REPTILES Common garden skink Lampropholis guichenoti C, Res Diamond python Morelia spilota Res Eastern blue tongue lizard Tilliqua scincoides C, Res Eastern long - necked turtle Chelondina longicollis Res Eastern small - eyed snake Cryptophis nigrescens Res Eastern tiger snake Notechis scutatus Res (escarpment) Eastern water dragon Physignathus lesurerii C, Res Eastern water skink Eulamprus quoyii C, Res Jacky dragon Amphibolurus muricatus Res Golden crowned snake Cacophis squamulosus Res Macquarie turtle Emydura macquarii Res Marsh (swamp) snake Hemiaspis signata Res Mustard bellied snake Drysdalia rhodogaster V Red - bellied black snake Pseudechis porphriacus Res Sheoak skink Cyclodomorphus michaeli Res Three - toed skink Saiphos equalis Res Weasel skink Saproscincus mustelinus Res MAMMALS Bush rat Rattus fuscipes -
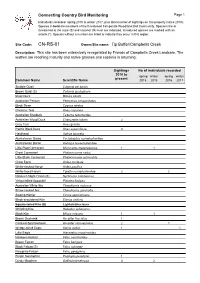
CN-RS-01 Owner/Site Name: Tip Buffer/Campbells Creek
Connecting Country Bird Monitoring Page 1 Individuals recorded spring 2015 to winter 2017, plus total number of sightings on this property (since 2010). Species in bold are members of the threatened Temperate Woodland Bird Community. Species rare or threatened at the state (S) and national (N) level are indicated. Introduced species are marked with an asterix (*). Species without a number are listed to indicate they occur in this region. Site Code: CN-RS-01 Owner/Site name: Tip Buffer/Campbells Creek Description: This site has been extensively revegetated by Friends of Campbells Creek Landcare. The wattles are reaching maturity and native grasses and cassinia is returning. Sightings No of individuals recorded 2010 to spring winter spring winter present Common Name Scientific Name 2015 2016 2016 2017 Stubble Quail Coturnix pectoralis Brown Quail (S) Coturnix ypsilophora Musk Duck Biziura lobata Australian Pelican Pelecanus conspicillatus Black Swan Cygnus atratus Chestnut Teal Anas castanea Australian Shelduck Tadorna tadornoides Australian Wood Duck Chenonetta jubata 2 Grey Teal Anas gracilis Pacific Black Duck Anas superciliosa 3 Hardhead Aythya australis Australasian Grebe Tachybaptus novaehollandiae Australasian Darter Anhinga novaehollandiae Little Pied Cormorant Microcarbo melanoleucos 1 1 Great Cormorant Phalacrocorax carbo Little Black Cormorant Phalacrocorax sulcirostris Great Egret Ardea modesta White-necked Heron Ardea pacifica White-faced Heron Egretta novaehollandiae 2 2 Nankeen Night-Heron (S) Nycticorax caledonicus Yellow-billed -

Activity, Habitat Use and the First Evidence of Lingual Lure Behavior in a South American Snake
SALAMANDRA 56(1): 39–47 Activity, habitat use and lingual lure behavior in a South American snakeSALAMANDRA 15 February 2020 ISSN 0036–3375 German Journal of Herpetology A fisherman’s tale: Activity, habitat use and the first evidence of lingual lure behavior in a South American snake Conrado Mario-da-Rosa1, Arthur Diesel Abegg2,3, Leandro Malta-Borges1, Alexandre Ferreira Righi4, Paulo Sérgio Bernarde5, Sonia Zanini Cechin1 & Tiago Gomes dos Santos1,6 1) Programa de Pós-Graduação em Biodiversidade Animal, Universidade Federal de Santa Maria, Av. Roraima, 1000, Santa Maria, Rio Grande do Sul, Brazil 2) Laboratório Especial de Coleções Zoológicas, Instituto Butantan, Av. Vital Brasil, 1500, São Paulo, São Paulo, Brazil 3) Programa de Pós-Graduação em Zoologia, Instituto de Biociências, Universidade de São Paulo, Travessa 14, Rua do Matão, 321, 05508-090, São Paulo, São Paulo, Brazil 4) Museu de História Natural e Jardim Botânico da Universidade Federal de Minas Gerais, R. Gustavo da Silveira, 1035, Belo Horizonte, Minas Gerais, Brazil 5) Laboratório de Herpetologia, Centro Multidisciplinar, Campus Floresta, Universidade Federal do Acre, Cruzeiro do Sul, Acre, Brazil 6) Universidade Federal do Pampa, Campus São Gabriel, Av. Antônio Trilha, 1847, São Gabriel, Rio Grande do Sul, Brazil Corresponding author: Conrado Mario-da-Rosa, e-mail: [email protected] Manuscript received: 23 August 2019 Accepted: 25 November 2019 by Arne Schulze Abstract. Literature data suggests that Thamnodynastes strigatus (Serpentes, Dipsadidae) is a snake that actively forages for anurans near waterbodies, using several microhabitats for this activity (e.g., shrubs, soil, and water). However, herein we present dissonant data previously known to the species, both concerning type of prey and foraging strategy. -

Technique for Measuring Speed and Visual Motion Sensitivity in Lizards
Psicológica (2008), 29, 133-151. Technique for measuring speed and visual motion sensitivity in lizards Kevin L. Woo* and Darren Burke Macquarie University (Australia) Testing sensory characteristics on herpetological species has been difficult due to a range of properties related to physiology, responsiveness, performance ability, and the type of reinforcer used. Using the Jacky lizard as a model, we outline a successfully established procedure in which to test the visual sensitivity to motion characteristics. We incorporated modifications to traditional operant paradigms by using three video playback systems to deliver random-dot kinematogram motion stimuli coupled with salient computer-animated secondary reinforcers representative of biologically important appetitive stimuli. This procedure has the capacity to test other visual aspects in lizards as well as other non- human species using video playback and computer-animation techniques as experimental tools. Psychophysical studies that investigate the visual systems in various taxa are often challenging and require tedious trial-and-error testing before establishing a successful procedure (Ozlak & Wickens, 1999; Feldman & Balch, 2004). Some protocols have already been established for successful replication on similar taxa, or can provide a basis in which to test the sensory system in completely different species. However, not all available procedures are applicable to every species. Researchers must then develop a new method using basic conditioning paradigms. Limitations are also governed by the way in which the species is motivated to respond. These problematic features can be a function of the biological nature of the * Kevin L. Woo was supported by the Centre for the Integrative Study of Animal Behaviour postgraduate award, and the Australian Research Council. -

Use of Backpack Radio-Transmitters on Lizards of the Genus Aspidocelis (Squamata: Teiidae)
Other Contributions Miscellaneous Notes Use of backpack radio-transmitters on lizards of the genus Aspidocelis (Squamata: Teiidae) Radio-transmitters are used widely in field studies when tracking animals under natural conditions, especially when the spatial or temporal location of individuals provides information for behavioral studies and the weight of the transmitters has minimal adverse effects on their behavior. Radio telemetry has been used in ecological studies in lizards (e.g., Richmond, 1998; Warner et al., 2006; García-Bastida et al., 2012) and various methods have been proposed for securing the radio-transmitters (e.g., Fisher and Mut, 1995; Goodman, 2005; Goodman et al., 2009); not all methods, however, are suitable for the different sizes, shapes, or habits of the lizards under study. Lizards of the genus Aspidoscelis are slender, highly active, and agile, so the use of external equipment that is too heavy can compromise their activity and survival. For example, species in this genus usually seek refuge among rocks and holes in the ground, and the addition of an object strapped to their body might limit their mobility to enter or leave the shelters. Moreover, during annual periods of inactivity these lizards become fossorial in their habits, and therefore require the use of some lightweight material for attaching an external transmitter. Herein we describe a simple, inexpensive, and lightweight material that can be used for attaching an external radio-transmitter for telemetry field studies on lizards. This method involves a modification of that proposed by Gerner (2008) for the gecko Phelsuma guentheri, and is based on the use of a simple backpack, of low cost and high reliability, in which light-weight material is used.