82864099.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Book CMU 5(2)
CMU. Journal (2006) Vol. 5(2) 169 Survey and Herbarium Specimens of Medicinal Vascular Flora of Doi Suthep-Pui Somporn Putiyanan1* and J.F. Maxwell2 1 Department of Pharmaceutical Science, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand 2 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand *Corresponding author. E-mail: [email protected] ABSTRACT The herbarium includes over 9,285 specimens from 238 families (270 fam. in the word) in medicinal plant herbarium, Faculty of Pharmacy, Chiang Mai University. From July 1987 to September 1991, a total of 2,044 species have been collected from Doi Suthep- Pui National Park, some of which are of considerable economic, medicinal and botanical interest. Vascular plants in this national park comprise of 193 of the 228 known families of vascular plants in Thailand, including a new family record for the flora of Thailand (Lardizabalaceae), eleven species new records for Thailand, three emended descriptions, two new combinations, and at least two species, with several others that are probably undescribed and new to science. The lowland, mostly disturbed forests up to 350-950 m. elevation, are of two deciduous facies, viz., dipterocarp-oak and mixed (former teak) forest. Elevations above this to the summit of Doi Suthep (c.1,620 m.) and Doi Pui (c.1,685 m.) are primary evergreen (monsoon) with some residual pine on some of the ridges. There is a distinct dry season (December-May) during which there are fires and many of the lowland species flower and fruit, many become leafless while in the evergreen areas, there is no specific flowering or fruiting season, that is, the phenologies of the plants in this habitat vary according to each species throughout the year. -

Bulletin of Natural History ®
FLORI'IDA MUSEUM BULLETIN OF NATURAL HISTORY ® A MIDDLE EOCENE FOSSIL PLANT ASSEMBLAGE (POWERS CLAY PIT) FROM WESTERN TENNESSEE DavidL. Dilcher and Terry A. Lott Vol. 45, No. 1, pp. 1-43 2005 UNIVERSITY OF FLORIDA GAINESVILLE - The FLORIDA MUSEUM OF NATURAL HiSTORY is Florida«'s state museum of natural history, dedicated to understanding, preser¥ingrand interpreting].biologica[1 diversity and culturafheritage. The BULLETIN OF THE FLORIDA- MUSEUM OF NATURAL HISTORY is a peer-reviewed publication thatpziblishes.the result5 of origifial reseafchin zodlogy, botany, paleontology, and archaeology. Address all inquiries t6 the Managing Editor ofthe Bulletin. Numbers,ofthe Bulletin,afe,published,at itregular intervals. Specific volumes are not'necessarily completed in anyone year. The end of a volume willl·be noted at the foot of the first page ofthe last issue in that volume. Richard Franz, Managing Editor Erika H. Simons, Production BulletinCommittee Richard Franz,,Chairperson Ann Cordell Sarah Fazenbaker Richard Hulbert WilliamMarquardt Susan Milbrath Irvy R. Quitmyer - Scott Robinson, Ex 01#cio Afember ISSN: 0071-6154 Publication Date: October 31,2005 Send communications concerning purchase or exchange of the publication and manustfipt queries to: Managing Editor of the BULLETIN Florida MuseumofNatural-History University offlorida PO Box 117800 Gainesville, FL 32611 -7800 U.S.A. Phone: 352-392-1721 Fax: 352-846-0287 e-mail: [email protected] A MIDDLE EOCENE FOSSIL PLANT ASSEMBLAGE (POWERS CLAY PIT) FROM WESTERN TENNESSEE David L. Dilcher and Terry A. Lottl ABSTRACT Plant megafossils are described, illustrated and discussed from Powers Clay Pit, occurring in the middle Eocene, Claiborne Group of the Mississippi Embayment in western Tennessee. -

De Novo Transcriptome Analysis of Dalbergia Odorifera T
Article De Novo Transcriptome Analysis of Dalbergia odorifera T. Chen (Fabaceae) and Transferability of SSR Markers Developed from the Transcriptome Fu-Mei Liu 1,2 , Zhou Hong 3,*, Zeng-Jiang Yang 3, Ning-Nan Zhang 3, Xiao-Jin Liu 3 and Da-Ping Xu 3 1 State Key Laboratory of Tree Genetics and Breeding, Northeast Forestry University, Harbin 150040, China; [email protected] 2 The Experimental Centre of Tropical Forestry, Chinese Academy of Forestry, Pingxiang 532600, China 3 Research Institute of Tropical Forestry, Chinese Academy of Forestry, Longdong, Guangzhou 510520, China; [email protected] (Z.-J.Y.); [email protected] (N.-N.Z.); [email protected] (X.-J.L.); [email protected] (D.-P.X.) * Correspondence: [email protected]; Tel.: +86-20-8703-1037 Received: 15 December 2018; Accepted: 24 January 2019; Published: 26 January 2019 Abstract: Dalbergia odorifera T. Chen (Fabaceae), indigenous to Hainan Island, is a precious rosewood (Hainan hualimu) in China. However, only limited genomic information is available which has resulted in a lack of molecular markers, limiting the development and utilization of the germplasm resources. In this study, we aim to enrich genomic information of D. odorifera, and develop a series of transferable simple sequence repeat (SSR) markers for Dalbergia species. Therefore, we performed transcriptome sequencing for D. odorifera by pooling leaf tissues from three trees. A dataset of 138,516,418 reads was identified and assembled into 115,292 unigenes. Moreover, 35,774 simple sequence repeats (SSRs) were identified as potential SSR markers. A set of 19 SSR markers was successfully transferred across species of Dalbergia odorifera T. -

ESJ67 Program Web.Pdf
CONTENTS 㣐⠓傈玎嚊殛.FFUJOH4DIFEVMF 㣐⠓傈玎♧鋮邌 5JNFUBCMF 长周ⰻ٥岣䠐✲갪٥傈劤欰䡾㷕⠓㣐⠓鋉 (FOFSBMJOTUSVDUJPOT /PUJDFTGPSQBSUJDJQBOUT #ZMBXTGPSUIF"OOVBM.FFUJOHTPGUIF&DPMPHJDBM4PDJFUZPG+BQBO NBQTBOENBJOWFOVF 㜥周ⰻ"DDFTT⠓٥㖑㔳٥أإؙ، -FDUVSFTCZBXBSESFDJQJFOUT 颣鎸䙀闌怴"XBSEDFSFNPOZ」㷕⠓颣ぐ颣䱇颣䒭٥ Ⱅ闌怴⠓1VCMJDMFDUVSFT 欰䡾㷕闌䏟ו欰䡾㷕闌䏟պ٥ֿ،صُآךז٦涪邌⠓չةأه넝吤欰 1PTUFSQSFTFOUBUJPOTCZIJHITDIPPMTUVEFOUT &DPMPHZDPVSTFGPSIJHITDIPPMTUVEFOUT &DPMPHZDPVSTFGPSDIJMESFO 嚊銲4ZNQPTJVNTيؐآهٝء 荈歋꧊⠓嚊銲8PSLTIPQT 嚊銲'PSVNTيؿؓ٦ٓ 〡걧涪邌♧鋮 0SBMQSFTFOUBUJPOT ٦涪邌♧鋮ةأه 1PTUFSQSFTFOUBUJPOT ٦涪邌♧鋮ةأه넝吤欰 1PTUFSQSFTFOUBUJPOTCZIJHITDIPPMTUVEFOUT 歗罏♧鋮1SFTFOUFSTBOE0SHBOJ[FST*OEFY⟰涪邌罏٥ "EWFSUJTFNFOU 额♧鋮٥䎢デ4QPOTPST⼿ 周ⰻ׀ך痥㔐傈劤欰䡾㷕⠓㣐⠓䎃剢٥䀤㿊 *NGPSNBUJPOPGUIFUI"OOVBM.FFUJOHPG&4+ JO0LBZBNB ٥㣐⠓㹋遤㨻㆞⠓せ砢$PNNJUUFFT⠓㣐⠓⟰歗㨻㆞ ׅկת鋮ְֽ׀דآ٦لي٦م⠓㣐כ姻铎邌ךيؚٓٗف 5IFFSSBUBPGUIFQSPHSBNBSFEJTDMPTFEJOUIF&4+XFCTJUF 㷕⠓ꞿ⽑鿇㙹㣕龤 㣐⠓⠓ꞿ傈ꅿ鰛僇խ 㣐⠓㹋遤㨻㆞ꞿ堀劤㉔〷խ㣐⠓⟰歗㨻㆞ꞿⰻ嵲⤥➜ 宓宵宨家宬宧宨宱宷季孲季宍宲宷室宵宲季官宕宄宅守 宆宫室宬宵季宲宩季宒宵宪室宱宬宽宬宱宪季宆宲宰宰宬宷宷宨宨季孲季宗宨宵宸室宮宬季宋完宑宒 宖宨宦宵宨宷室宵宼季宊宨宱宨宵室宯季宲宩季宒宵宪室宱宬宽宬宱宪季宆宲宰宰宬宷宷宨宨季孲季宋宬宵宲家宫宬季宋宄宖宋完宐宒宗宒 宆宫室宬宵季宲宩季宄宱宱宸室宯季宐宨宨宷宬宱宪季宓宯室宱宬宱宪季宆宲宰宰宬宷宷宨宨季孲季宖宫宸宱家宸宮宨季官宗宖官宐完 㣐⠓傈玎嚊殛.FFUJOH4DIFEVMF Ӫぐ珏㨻㆞⠓$PNNJUUFFNFFUJOHT 宏8FE 㣐⠓⟰歗㨻㆞⠓3PPN) 㢩勻珏㉏겗嗚鎢⡲噟鿇⠓3PPN* 欰䡾禸盖椚㼔㨻㆞⠓3PPN+ 㼛勻鎘歗㼔㨻㆞⠓3PPN& 荈搫⥂隊㼔㨻㆞⠓3PPN' ⥂Ⰻ欰䡾㷕灇瑔ⴚ遤⼿陽⠓3PPN) ٍؗٔ،佄䴂㼔㨻㆞⠓3PPN* ꅿ㢩㸜Ⰻ盖椚㨻㆞⠓3PPN+ &DPMPHJDBM3FTFBSDIⴚ遤⼿陽⠓3PPN' 傈劤欰䡾㷕⠓钞ⴚ遤⼿陽⠓3PPN) 欰䡾㷕侄肪㼔㨻㆞⠓3PPN* 㣐鋉垷ꞿ劍欰䡾㷕㼔㨻㆞⠓3PPN+ ➿陽㆞⠓3PPN# Ӫ筨⠓䱇颣䒭「颣闌怴(FOFSBMNFFUJOH "XBSEDFSFNPOZ -FDUVSFTCZBXBSESFDJQJFOUT 㕼4BU Room A / ˊ 筨⠓(FOFSBMNFFUJOH FDUVSFTCZBXBSESFDJQJFOUT-颣鎸䙀闌怴"XBSEDFSFNPOZ٥」䱇颣䒭٥ ˊ &3锷俑颣薉铂〡걧涪邌颣⸆⸤颣㤺⸠ ꈿ加 颣 խխ ˊ 㣐䃊颣㹧㖑颣欰䡾㷕⠓颣 ♧菙闌怴ي٥ؿؓ٦ٓ⠓荈歋꧊يؐآهٝءӪ -

The Archaeobotany of Khao Sam Kaeo and Phu Khao Thong: the Agriculture of Late Prehistoric Southern Thailand (Volume 1)
The Archaeobotany of Khao Sam Kaeo and Phu Khao Thong: The Agriculture of Late Prehistoric Southern Thailand (Volume 1) Cristina Castillo Institute of Archaeology University College London Thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy of University College London 2013 Declaration I hereby declare that this dissertation consists of original work undertaken by the undersigned. Where other sources of information have been used, they have been acknowledged. Cristina Castillo October 2013 Institute of Archaeology, UCL 2 Abstract The Thai-Malay Peninsula lies at the heart of Southeast Asia. Geographically, the narrowest point is forty kilometres and forms a barrier against straightforward navigation from the Indian Ocean to the South China Sea and vice versa. This would have either led vessels to cabotage the southernmost part of the peninsula or portage across the peninsula to avoid circumnavigating. The peninsula made easy crossing points strategic locations commercially and politically. Early movements of people along exchange routes would have required areas for rest, ports, repair of boats and replenishment of goods. These feeder stations may have grown to become entrepôts and urban centres. This study investigates the archaeobotany of two sites in the Thai-Malay Peninsula, Khao Sam Kaeo and Phu Khao Thong. Khao Sam Kaeo is located on the east whereas Phu Khao Thong lies on the west of the peninsula and both date to the Late Prehistoric period (ca. 400-100 BC). Khao Sam Kaeo has been identified as the earliest urban site from the Late Prehistoric period in Southeast Asia engaged in trans-Asiatic exchange networks. -

Quercus Cerris L.) Populations
PATTERN OF GENETIC DIVERSITY IN TURKEY OAK (QUERCUS CERRIS L.) POPULATIONS A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY YELĠZ TÜMBĠLEN ÖZER IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY JULY 2014 Approval of the Thesis PATTERN OF GENETIC DIVERSITY IN TURKEY OAK (QUERCUS CERRIS L.) POPULATIONS submitted by YELİZ TÜMBİLEN ÖZERin partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology Department, Middle East Technical University by, Prof. Dr. Canan Özgen _______________ Dean, Graduate School of Natural and Applied Sciences Prof. Dr. Orhan Adalı _______________ Head of the Department, Biology Prof. Dr. Zeki Kaya _______________ Supervisor, Biology Dept., METU Examining Committee Members: Prof. Dr. Musa Doğan _______________ Biology Dept., METU Prof.Dr. Zeki Kaya _______________ Biology Dept., METU Prof. Dr. Hayri Duman _______________ Biology Dept., Gazi University Assoc. Prof. Dr. Sertaç Önde _______________ Biology Dept., METU Assist. Prof. Dr. AyĢegül Birand _______________ Biology Dept., METU Date: 03.07.2014 iii I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name : Yeliz TÜMBĠLEN ÖZER Signature: iv ABSTRACT PATTERN OF GENETIC DIVERSITY IN TURKEY OAK (QUERCUS CERRIS L.) POPULATIONS TÜMBĠLEN ÖZER, Yeliz Ph D., Department of Biology Supervisor: Prof. Dr. Zeki KAYA July 2014, 119 pages Quercus cerris L. -

In Situ Dark Adaptation Enhances the Efficiency of DNA Extraction
Article In Situ Dark Adaptation Enhances the Efficiency of DNA Extraction from Mature Pin Oak (Quercus palustris) Leaves, Facilitating the Identification of Partial Sequences of the 18S rRNA and Isoprene Synthase (IspS) Genes Csengele E. Barta *, Bethany Bolander, Steven R. Bilby, Jeremy H. Brown, Reid N. Brown, Alexander M. Duryee, Danielle R. Edelman, Christina E. Gray, Chandler Gossett, Amie G. Haddock, Mackenzie M. Helsel, Alyssa D. Jones, Marissa E. Klingseis, Kalif Leslie, Edward W. Miles and Rachael A. Prawitz Department of Biology, Missouri Western State University, 4525 Downs Drive, Agenstein-Remington Halls, St. Joseph, MO 64507, USA; [email protected] (B.B.); [email protected] (S.R.B.); [email protected] (J.H.B.); [email protected] (R.N.B.); [email protected] (A.M.D.); [email protected] (D.R.E.); [email protected] (C.E.G.); [email protected] (C.G.); [email protected] (A.G.H.); [email protected] (M.M.H.); [email protected] (A.D.J.); [email protected] (M.E.K.); [email protected] (K.L.); [email protected] (E.W.M.); [email protected] (R.A.P.) * Correspondence: [email protected]; Tel.: +1-816-271-4334 Received: 28 August 2017; Accepted: 19 October 2017; Published: 24 October 2017 Abstract: Mature oak (Quercus spp.) leaves, although abundantly available during the plants’ developmental cycle, are rarely exploited as viable sources of genomic DNA. These leaves are rich in metabolites difficult to remove during standard DNA purification, interfering with downstream molecular genetics applications. The current work assessed whether in situ dark adaptation, to deplete sugar reserves and inhibit secondary metabolite synthesis could compensate for the difficulties encountered when isolating DNA from mature leaves rich in secondary metabolites. -

FAGACEAE 1. FAGUS Linnaeus, Sp. Pl. 2: 997. 1753
Flora of China 4: 314–400. 1999. 1 FAGACEAE 壳斗科 qiao dou ke Huang Chengjiu (黄成就 Huang Ching-chieu)1, Zhang Yongtian (张永田 Chang Yong-tian)2; Bruce Bartholomew3 Trees or rarely shrubs, monoecions, evergreen or deciduous. Stipules usually early deciduous. Leaves alternate, sometimes false-whorled in Cyclobalanopsis. Inflorescences unisexual or androgynous with female cupules at the base of an otherwise male inflorescence. Male inflorescences a pendulous head or erect or pendulous catkin, sometimes branched; flowers in dense cymules. Male flower: sepals 4–6(–9), scalelike, connate or distinct; petals absent; filaments filiform; anthers dorsifixed or versatile, opening by longitudinal slits; with or without a rudimentary pistil. Female inflorescences of 1–7 or more flowers subtended individually or collectively by a cupule formed from numerous fused bracts, arranged individually or in small groups along an axis or at base of an androgynous inflorescence or on a separate axis. Female flower: perianth 1–7 or more; pistil 1; ovary inferior, 3–6(– 9)-loculed; style and carpels as many as locules; placentation axile; ovules 2 per locule. Fruit a nut. Seed usually solitary by abortion (but may be more than 1 in Castanea, Castanopsis, Fagus, and Formanodendron), without endosperm; embryo large. Seven to 12 genera (depending on interpretation) and 900–1000 species: worldwide except for tropical and S Africa; seven genera and 294 species (163 endemic, at least three introduced) in China. Many species are important timber trees. Nuts of Fagus, Castanea, and of most Castanopsis species are edible, and oil is extracted from nuts of Fagus. Nuts of most species of this family contain copious amounts of water soluble tannin. -
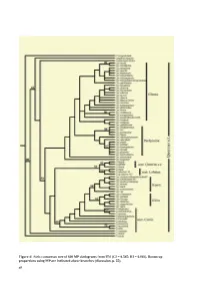
Taxonomy and Systematics of Quercus Subgenus Cyclobalanopsis
Figure 4/ Strict consensus tree of 600 MP cladograms from ITS (CI = 0.545; RI = 0.803). Bootstrap proportions using MP are indicated above branches (discussion, p. 55). 48 Taxonomy and Systematics of Quercus Subgenus Cyclobalanopsis Min Deng1, Zhekun Zhou2*, Qiansheng Li3 2. Xishuangbanna Tropical Botanical Garden, 3. School of Ecology, the Chinese Academy of Sciences, Shanghai Institute of Technology, Kunming, 650223, China. 100 Haiquan Rd., Shanghai, 201418, China 1. Chenshan Plant Sciences Research Center, the Chinese Academy of Sciences, Chenshan Botanical Garden, 3888 ChenHua Rd., Shanghai, 201602, China. Phone: +86-21- 57 79 93 82; Fax: + 86-21-67657811. [email protected]; ABSTRACT Quercus subgenus Cyclobalanopsis is one of the dominant woody plant groups in E and SE Asia, but comprehensive studies on its systematics and taxonomy are limited. In this study, we compared the leaf epidermal and acorn features of 52 species in subgenus Cyclobalanopsis and 15 species from Quercus subgenus Quercus. We also studied molecular phylogeny using DNA sequences from the nuclear ribosomal internal transcribed spacer (ITS) region and the chloroplast psbA–trnH and trnT–trnL regions. Both the leaf epidermal and acorn features indicated ʏve morphologically distinct groups in Cyclobalanopsis: 1) Gilva group (fused stellate trichomes with compound trichome base); 2) Kerrii group (with fasciculate trichomes, and radicles emerging from the basal seed scar; 3) Pachyloma group (papillae on epidermal cells, but glabrous when mature and densely yellowish woolly on the cupule walls); 4) Jenseniana group (lamellae mostly fused to the cupule wall with only the rims free); 5) Glauca group (appressed-lateral-attached trichomes). -

2010-41 Panadda Larpkern (INA).Pdf
Philosophiae Doctor (P <]hYjle]flg^=[gdg_qYf\FYlmjYdJ]kgmj[]EYfY_]e]fl Fgjo]_aYfMfan]jkalqg^Da^]K[a]f[]kMfan]jkal]l]l^gjeadb¬ Woody species regeneration and diversity in a seasonally dry forest in northeastern h D) Thesis 2010:41 Thailand J]cjmll]jaf_g_\an]jkal]l`gklj]Yjl]ja]fl¬jc]mlkYllkcg_af¬j\¬klj] Thailand Panadda Larpkern %g_Zagnal]fkcYh Woodyspeciesregenerationanddiversityinaseasonallydry forestinnortheasternThailand #)0322#0',%-%"'4#01'2#2&-120#02#0'#,20)#321221)-%',0"120#&'*," &'*-1-.&'#-!2-0&'1 ,""0.)#0, #.2T-$!-*-%7," 230*0#1-30!#!,%#+#,2 -05#%',$,'4#01'27-$'$#%!'#,!#1 &1TRSR '1,3+ #0TRSRSVS -%% SWRUVSXXY -%3 [YZVZTVWYWVR[WSVV PhD supervisors: Professor Stein R. Moe Department of Ecology and Natural Resource Management Norwegian University of Life Sciences P.O. Box 5003 NO-1432 Ås, Norway Professor Ørjan Totland Department of Ecology and Natural Resource Management Norwegian University of Life Sciences P.O. Box 5003 NO-1432 Ås, Norway Adjunction committee: Dr. Graciela M. Rusch Norwegian Institute for Nature Research Terrestrisk avd. NO-7485 Trondheim, Norway Professor Jens M. Olesen Department of Biological Sciences Aarhus University DK-8000, Denmark Professor Tron Eid Department of Ecology and Natural Resource Management Norwegian University of Life Sciences P.O. Box 5003 NO-1432 Ås, Norway ii Acknowledgement This thesis is submitted in partial fulfillment of the requirements for the Philosophiae Doctor (PhD) degree at the Norwegian University of Life Sciences (UMB). The PhD scholarship was covered by the quota program of the Norwegian State Educational Loan Fund. I would like also to thank the Department of Ecology and Natural Resource Management (INA) for providing me additional financial support. -

An Approach from Phylogenetic and Functional Signals in Beneficial Attributes of Tree Species
Multifaceted ecosystem services provided by tree communities: an approach from phylogenetic and functional signals in beneficial attributes of tree species 著者 Oka Chihiro 学位授与機関 Tohoku University 学位授与番号 11301甲第18786号 URL http://hdl.handle.net/10097/00125750 博 士 論 文 Multifaceted ecosystem services provided by tree communities: an approach from phylogenetic and functional signals in beneficial attributes of tree species (樹木群集による多面的生態系サービス供給: 樹種の有用性にみられる系統的・機能的シグナルに基づくアプローチ) 平成30年度 東北大学大学院生命科学研究科 生態システム生命科学専攻 岡 千尋 Table of Contents Abstract…………………………………………………………………………………………...2 Chapter 1. General introduction………………………………………………………………….5 Chapter 2. Phylogenetic clustering in beneficial attributes of tree species directly linked to provisioning, regulating and cultural ecosystem services……………………………………….12 Chapter 3. Linking functional traits to ecosystem services: quantification of important traits for provisioning, regulating, and cultural benefits of tree species…………………………………..38 Chapter 4. Importance of species identity and community composition for ecosystem services of tree communities…………………………………………………………………………………54 Chapter 5. General discussion…………………………………………………………………..70 Acknowledgements……………………………………………………………………………..74 Supplementary information……………………………………………………………………75 1 Abstract Organisms and the communities play essential roles for ecosystem functions and services (i.e., human benefits from ecosystems). Effects of organisms and the communities on ecosystem functions/services are mediated by functional traits of organisms. Functional -

Seasonally Dry Tropical Forests in Continental Southeast Asia Structure, Composition, and Dynamics
1 Seasonally Dry Tropical Forests in Continental Southeast Asia Structure, Composition, and Dynamics Sarayudh Bunyavejchewin, Patrick J. Baker, and Stuart J. Davies he forests of continental Southeast Asia make up a significant portion of the Indo-Burma biodiversity hotspot (see McShea and Davies, this volume). These Tdiverse forests are under severe threat from both land use and climate change, and urgently need to be more sustainably managed. This management of seasonally dry forests in Southeast Asia needs to be based on a sound understanding of the ecol- ogy of natural forests, the habitat requirements of their constituent species, and the response of these systems to natural disturbance dynamics. Natural forest management strategies will help protect habitat for wildlife, will limit the impact of nonnatural dis- turbances, and will lead to opportunities for restoring degraded lands to functional for- ests. An important first step in this process is to develop a much more refined descrip- tion of the forests with a clear understanding of what controls their spatial variation in structure and composition across the region. The vegetation types and ecoregions described in recent mapping and conservation assessments in continental Southeast Asia—for example, Blasco et al. (1996) and Wikramanayake et al. (2002)—are useful for broad-scale threat assessment and priority setting; however, for the active forest management that is required in much of the region now, we need detailed understand- ing of the ecology and dynamics of specific forest types. Our research aims to understand the controls on spatial distributions and temporal dynamics of seasonally dry tropical forest (SDTF) formations across the region.