Book CMU 5(2)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Annotated Checklist of the Angiospermic Flora of Rajkandi Reserve Forest of Moulvibazar, Bangladesh
Bangladesh J. Plant Taxon. 25(2): 187-207, 2018 (December) © 2018 Bangladesh Association of Plant Taxonomists AN ANNOTATED CHECKLIST OF THE ANGIOSPERMIC FLORA OF RAJKANDI RESERVE FOREST OF MOULVIBAZAR, BANGLADESH 1 2 A.K.M. KAMRUL HAQUE , SALEH AHAMMAD KHAN, SARDER NASIR UDDIN AND SHAYLA SHARMIN SHETU Department of Botany, Jahangirnagar University, Savar, Dhaka 1342, Bangladesh Keywords: Checklist; Angiosperms; Rajkandi Reserve Forest; Moulvibazar. Abstract This study was carried out to provide the baseline data on the composition and distribution of the angiosperms and to assess their current status in Rajkandi Reserve Forest of Moulvibazar, Bangladesh. The study reports a total of 549 angiosperm species belonging to 123 families, 98 (79.67%) of which consisting of 418 species under 316 genera belong to Magnoliopsida (dicotyledons), and the remaining 25 (20.33%) comprising 132 species of 96 genera to Liliopsida (monocotyledons). Rubiaceae with 30 species is recognized as the largest family in Magnoliopsida followed by Euphorbiaceae with 24 and Fabaceae with 22 species; whereas, in Lilliopsida Poaceae with 32 species is found to be the largest family followed by Cyperaceae and Araceae with 17 and 15 species, respectively. Ficus is found to be the largest genus with 12 species followed by Ipomoea, Cyperus and Dioscorea with five species each. Rajkandi Reserve Forest is dominated by the herbs (284 species) followed by trees (130 species), shrubs (125 species), and lianas (10 species). Woodlands are found to be the most common habitat of angiosperms. A total of 387 species growing in this area are found to be economically useful. 25 species listed in Red Data Book of Bangladesh under different threatened categories are found under Lower Risk (LR) category in this study area. -

Bulletin of Natural History ®
FLORI'IDA MUSEUM BULLETIN OF NATURAL HISTORY ® A MIDDLE EOCENE FOSSIL PLANT ASSEMBLAGE (POWERS CLAY PIT) FROM WESTERN TENNESSEE DavidL. Dilcher and Terry A. Lott Vol. 45, No. 1, pp. 1-43 2005 UNIVERSITY OF FLORIDA GAINESVILLE - The FLORIDA MUSEUM OF NATURAL HiSTORY is Florida«'s state museum of natural history, dedicated to understanding, preser¥ingrand interpreting].biologica[1 diversity and culturafheritage. The BULLETIN OF THE FLORIDA- MUSEUM OF NATURAL HISTORY is a peer-reviewed publication thatpziblishes.the result5 of origifial reseafchin zodlogy, botany, paleontology, and archaeology. Address all inquiries t6 the Managing Editor ofthe Bulletin. Numbers,ofthe Bulletin,afe,published,at itregular intervals. Specific volumes are not'necessarily completed in anyone year. The end of a volume willl·be noted at the foot of the first page ofthe last issue in that volume. Richard Franz, Managing Editor Erika H. Simons, Production BulletinCommittee Richard Franz,,Chairperson Ann Cordell Sarah Fazenbaker Richard Hulbert WilliamMarquardt Susan Milbrath Irvy R. Quitmyer - Scott Robinson, Ex 01#cio Afember ISSN: 0071-6154 Publication Date: October 31,2005 Send communications concerning purchase or exchange of the publication and manustfipt queries to: Managing Editor of the BULLETIN Florida MuseumofNatural-History University offlorida PO Box 117800 Gainesville, FL 32611 -7800 U.S.A. Phone: 352-392-1721 Fax: 352-846-0287 e-mail: [email protected] A MIDDLE EOCENE FOSSIL PLANT ASSEMBLAGE (POWERS CLAY PIT) FROM WESTERN TENNESSEE David L. Dilcher and Terry A. Lottl ABSTRACT Plant megafossils are described, illustrated and discussed from Powers Clay Pit, occurring in the middle Eocene, Claiborne Group of the Mississippi Embayment in western Tennessee. -

119-123 (2008) ว. วิทย. กษ. 39 : 3 (พเศษิ ) : 119-123 (2551)
Agricultural Sci. J. 39 : 3 (Suppl.) : 119-123 (2008) ว. วิทย. กษ. 39 : 3 (พเศษิ ) : 119-123 (2551) การยับยั้งเชื้อราสาเหตุโรคพืชดวยสารสกัดจากพืชในสกุล Rutaceae 16 ชนิด Antimicrobial activity of 16 plant extracts of the Rutaceae family against phytopathogenic fungi เนตรนภสิ เขียวขาํ 1, Harald Greger2 และ สมศิริ แสงโชต1ิ Netnapis Khewkhom1, Harald Greger2 and Somsiri Shangchote1 Abstract The lipophilic extract of 16 plant extracts of the Rutaceae family was selected for investigation of antifungal properties. Antifungal active compounds bioautography bioassays against Cladosporium herbarum have been detected. Comparative studies of Toddalia sp. (leaves), Limonia acidissima (leaves), Vepris bilocularis (leaves), Coleonema pulchellum (roots), Triphasia trifoliata (leaves), Pleiospermum alatum (leaves and stem), Acronychia pedunculata (leaves), and Atalantia sp. (leaves) extract showed clear inhibition zones on TLC plates against C. herbarum. In microdilution bioassay, the leaf extracts of Fortunella hindsii exhibited the strongest fungicidal activity with a MIC value at 312.5 μg/mL and showed an inhibition of spore germination at EC50 of 114 μg/mL for Botrytis cinerea. L. acidissima leaf extracts displayed a MIC of 1250 μg/mL and showed an inhibition of spore germination with EC50 0 values at 589 μg/mL for B. cinerea. The leaf extracts of two different collections of Glycosmis mauritiana (RUT 213/7) and (RUT 400) revealed clear differences for the antifungal activity: the EC50 value of the former was at 70 μg/mL, but 1249 μg/mL for the latter. G. mauritiana (RUT 213/7) revealed clear differences for the antifungal activity: the EC50 value at 70 μg/mL. Stem extracts of Pleiospermum alatum showed activities with values at EC50 262 μg/mL against B. -

De Novo Transcriptome Analysis of Dalbergia Odorifera T
Article De Novo Transcriptome Analysis of Dalbergia odorifera T. Chen (Fabaceae) and Transferability of SSR Markers Developed from the Transcriptome Fu-Mei Liu 1,2 , Zhou Hong 3,*, Zeng-Jiang Yang 3, Ning-Nan Zhang 3, Xiao-Jin Liu 3 and Da-Ping Xu 3 1 State Key Laboratory of Tree Genetics and Breeding, Northeast Forestry University, Harbin 150040, China; [email protected] 2 The Experimental Centre of Tropical Forestry, Chinese Academy of Forestry, Pingxiang 532600, China 3 Research Institute of Tropical Forestry, Chinese Academy of Forestry, Longdong, Guangzhou 510520, China; [email protected] (Z.-J.Y.); [email protected] (N.-N.Z.); [email protected] (X.-J.L.); [email protected] (D.-P.X.) * Correspondence: [email protected]; Tel.: +86-20-8703-1037 Received: 15 December 2018; Accepted: 24 January 2019; Published: 26 January 2019 Abstract: Dalbergia odorifera T. Chen (Fabaceae), indigenous to Hainan Island, is a precious rosewood (Hainan hualimu) in China. However, only limited genomic information is available which has resulted in a lack of molecular markers, limiting the development and utilization of the germplasm resources. In this study, we aim to enrich genomic information of D. odorifera, and develop a series of transferable simple sequence repeat (SSR) markers for Dalbergia species. Therefore, we performed transcriptome sequencing for D. odorifera by pooling leaf tissues from three trees. A dataset of 138,516,418 reads was identified and assembled into 115,292 unigenes. Moreover, 35,774 simple sequence repeats (SSRs) were identified as potential SSR markers. A set of 19 SSR markers was successfully transferred across species of Dalbergia odorifera T. -

Nazrin Full Phd Thesis (150246576
Maintenance and conservation of Dipterocarp diversity in tropical forests _______________________________________________ Mohammad Nazrin B Abdul Malik A thesis submitted in partial fulfilment of the degree of Doctor of Philosophy Faculty of Science Department of Animal and Plant Sciences November 2019 1 i Thesis abstract Many theories and hypotheses have been developed to explain the maintenance of diversity in plant communities, particularly in hyperdiverse tropical forests. Maintenance of the composition and diversity of tropical forests is vital, especially species of high commercial value. I focus on the high value dipterocarp timber species of Malaysia and Borneo as these have been extensive logged owing to increased demands from global timber trade. In this thesis, I explore the drivers of diversity of this group, as well as the determinants of global abundance, conservation and timber value. The most widely supported hypothesis for explaining tropical diversity is the Janzen Connell hypothesis. I experimentally tested the key elements of this, namely density and distance dependence, in two dipterocarp species. The results showed that different species exhibited different density and distance dependence effects. To further test the strength of this hypothesis, I conducted a meta-analysis combining multiple studies across tropical and temperate study sites, and with many species tested. It revealed significant support for the Janzen- Connell predictions in terms of distance and density dependence. Using a phylogenetic comparative approach, I highlight how environmental adaptation affects dipterocarp distribution, and the relationships of plant traits with ecological factors and conservation status. This analysis showed that environmental and ecological factors are related to plant traits and highlights the need for dipterocarp conservation priorities. -

A Study of Analgesic Activity of Methanol and Pet Ether Extracts of Barks of Stereospermum
“A Study of Analgesic Activity of Methanol and Pet ether Extracts of Barks of Stereospermum chelonoides” A DISSERTATION SUBMITTED TO THE DEPARTMENT OF PHARMACY, EAST WEST UNIVERSITY IN THE PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF BACHELOR OF PHARMACY Submitted By Lia Rose Merrry D. Cruze ID: 2012-1-70-005 Department of Pharmacy East West University Declaration by the Research Candidate I, Lia Rose Merry D. Cruze hereby declare that the dissertation entitled “A Study of Analgesic Activity of Methanol and Pet ether Extracts of Barks of Stereospermum chelonoides” submitted by me to the Department of Pharmacy, East West University, in the partial fulfillment of the requirement for the award of the degree Bachelor of Pharmacy is a complete record of original research work carried out by me during 2016, under the supervision and guidance of Meena Afroze Shanta, Senior Lecturer, Department of Pharmacy, East West University and the thesis has not formed the basis for the award of any other degree/diploma/fellowship or other similar title to any candidate of any university. _____________________________ Lia Rose Merry D. Cruze ID# 2012-1-70-005 Department of Pharmacy East West University, Dhaka, Bangladesh i Certificate by the Supervisor This is to certify that the thesis entitled “A Study of Analgesic Activities of Methanol and Pet ether Extracts of Barks of Stereospermum chelonoides” submitted to the Department of Pharmacy, East West University, in the partial fulfillment of the requirement for the degree of Bachelor of pharmacy was carried out by Lia Rose Merry D. Cruze, ID# 2012-1-70-005 in 2016, under the supervision and guidance of Meena Afroze Shanta. -

The Archaeobotany of Khao Sam Kaeo and Phu Khao Thong: the Agriculture of Late Prehistoric Southern Thailand (Volume 1)
The Archaeobotany of Khao Sam Kaeo and Phu Khao Thong: The Agriculture of Late Prehistoric Southern Thailand (Volume 1) Cristina Castillo Institute of Archaeology University College London Thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy of University College London 2013 Declaration I hereby declare that this dissertation consists of original work undertaken by the undersigned. Where other sources of information have been used, they have been acknowledged. Cristina Castillo October 2013 Institute of Archaeology, UCL 2 Abstract The Thai-Malay Peninsula lies at the heart of Southeast Asia. Geographically, the narrowest point is forty kilometres and forms a barrier against straightforward navigation from the Indian Ocean to the South China Sea and vice versa. This would have either led vessels to cabotage the southernmost part of the peninsula or portage across the peninsula to avoid circumnavigating. The peninsula made easy crossing points strategic locations commercially and politically. Early movements of people along exchange routes would have required areas for rest, ports, repair of boats and replenishment of goods. These feeder stations may have grown to become entrepôts and urban centres. This study investigates the archaeobotany of two sites in the Thai-Malay Peninsula, Khao Sam Kaeo and Phu Khao Thong. Khao Sam Kaeo is located on the east whereas Phu Khao Thong lies on the west of the peninsula and both date to the Late Prehistoric period (ca. 400-100 BC). Khao Sam Kaeo has been identified as the earliest urban site from the Late Prehistoric period in Southeast Asia engaged in trans-Asiatic exchange networks. -

82864099.Pdf
ORIGINAL RESEARCH published: 21 February 2017 doi: 10.3389/fpls.2017.00229 Introgression Threatens the Genetic Diversity of Quercus austrocochinchinensis (Fagaceae), an Endangered Oak: A Case Inferred by Molecular Markers Miao An 1, 2, Min Deng 1, 2*, Si-Si Zheng 1, 2, Xiao-Long Jiang 1, 2 and Yi-Gang Song 1, 2 1 Shanghai Key Laboratory of Plant Functional Genomics and Resources, Shanghai Chenshan Botanical Garden, Shanghai, China, 2 Shanghai Chenshan Plant Science Research Center, Chinese Academy of Sciences, Shanghai, China Natural introgression can cause negative effects where rare species experience genetic assimilation and invade by their abundant congeners. Quercus austrocochinchinensis and Q. kerrii (subgenus Cyclobalanopsis) are a pair of closely related species in the Edited by: Indo-China area. Morphological intermediates of the two species have been reported Scott V. Edwards, in this region. In this study, we used AFLP, SSR and two key leaf morphological Harvard University, USA diagnostic traits to study the two Q. austrocochinchinensis populations, two pure Reviewed by: Octavio Salgueiro Paulo, Q. kerrii and two putative hybrid populations in China. Rates of individual admixture Universidade de Lisboa, Portugal were examined using the Bayesian clustering programs STRUCTURE and NewHybrids, Yongpeng Ma, Kunming Institute of Botany (CAS), with no a priori species assignment. In total, we obtained 151 SSR alleles and China 781 polymorphic loci of AFLP markers. Population differentiation inferred by SSR *Correspondence: and AFLP was incoherent with recognized species boundaries. Bayesian admixture Min Deng analyses and principal coordinate analysis identified more hybrids and backcrossed [email protected] individuals than morphological intermediates in the populations. -

Forest Vegetation Cover in Binh Chau - Phuoc Buu Nature Reserve in Southern Vietnam
E3S Web of Conferences 175, 14016 (2020) https://doi.org/10.1051/e3sconf/202017514016 INTERAGROMASH 2020 Forest Vegetation Cover in Binh Chau - Phuoc Buu Nature Reserve in Southern Vietnam Viet Hung Dang¹ʾ²*, Alexander Potokin¹, Thi Lan Anh Dang², Thi Ha Nguyen², and Van Son Le³ 1 Saint-Petersburg State Forest Technical University, Instytutskiy 5U, 194021, St. Petersburg, Russia 2 Vietnam National University of Forestry - Dong Nai Campus, Vietnam, Dong Nai Province, Trang Bom District, Trang Bom town, Tran Phu st., 54 3 Binh Chau - Phuoc Buu Nature Reserve, Ba Ria - Vung Tau Province, Xuyen Moc District, Vietnam Abstract. Binh Chau - Phuoc Buu Nature Reserve is located in the tropical rainforest zone of southeast Vietnam. The obtained results from the study undertaken on the composition of plant species and forest vegetation in Binh Chau - Phuoc Buu Nature Reserve indicated a record of 743 species, 423 genera and 122 families that belongs to the three divisions of vascular plants. These includes: Polypodiophyta, Pinophyta and Magnoliophyta. Useful plants of 743 taxonomy species listed consists of 328 species of medicinal plants, 205 species of timber plants, 168 species of edible plants, 159 species of ornamental plants, 56 species of industrial plants, 10 species of fiber plants and 29 species of unknown use plants, respectively. During the duration of investigation, Nervilia aragoana Gaudich. was newly recorded in the forest vegetation of Binh Chau - Phuoc Buu Reserve. A variety of forest vegetations in the area under study is described. In this study, two major vegetation types of forest were identified in Binh Chau - Phuoc Buu Reserve. -

Plant Species Vulnerability to Climate Change in Peninsular Thailand
Utah State University DigitalCommons@USU CWEL Publications 2011 Plant Species vulnerability to climate change in peninsular Thailand. Y. Trisuart S. Fajendra Roger K. Kjelgren Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/cwel_pubs Recommended Citation Trisuart, Y.; Fajendra, S.; and Kjelgren, Roger K., "Plant Species vulnerability to climate change in peninsular Thailand." (2011). CWEL Publications. Paper 83. https://digitalcommons.usu.edu/cwel_pubs/83 This Article is brought to you for free and open access by DigitalCommons@USU. It has been accepted for inclusion in CWEL Publications by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Applied Geography 31 (2011) 1106e1114 Contents lists available at ScienceDirect Applied Geography journal homepage: www.elsevier.com/locate/apgeog Plant species vulnerability to climate change in Peninsular Thailand Yongyut Trisurat a,*, Rajendra P. Shrestha b, Roger Kjelgren c a Kasetsart University, Faculty of Forestry, 50 Ngamwongwan Road, Bangkok 10900, Thailand b School of Environment, Resources and Development, Asian Institute of Technology, Pathumthani 12120, Thailand c Department of Plants, Soils, and Climate, Utah State University, UT 84322, USA abstract Keywords: The objective of this research study was to evaluate the consequences of climate change on shifts in Climate change distributions of plant species and the vulnerability of the species in Peninsular Thailand. A sub-scene of Maxent the predicted climate in the year 2100, under the B2a scenario of the Hadley Centre Coupled Model, Peninsular Thailand version 3 (HadCM3), was extracted and calibrated with topographic variables. A machine learning Plant species Species distribution algorithm based on the maximum entropy theory (Maxent) was employed to generate ecological niche Species vulnerability models of 66 forest plant species from 22 families. -

FAGACEAE 1. FAGUS Linnaeus, Sp. Pl. 2: 997. 1753
Flora of China 4: 314–400. 1999. 1 FAGACEAE 壳斗科 qiao dou ke Huang Chengjiu (黄成就 Huang Ching-chieu)1, Zhang Yongtian (张永田 Chang Yong-tian)2; Bruce Bartholomew3 Trees or rarely shrubs, monoecions, evergreen or deciduous. Stipules usually early deciduous. Leaves alternate, sometimes false-whorled in Cyclobalanopsis. Inflorescences unisexual or androgynous with female cupules at the base of an otherwise male inflorescence. Male inflorescences a pendulous head or erect or pendulous catkin, sometimes branched; flowers in dense cymules. Male flower: sepals 4–6(–9), scalelike, connate or distinct; petals absent; filaments filiform; anthers dorsifixed or versatile, opening by longitudinal slits; with or without a rudimentary pistil. Female inflorescences of 1–7 or more flowers subtended individually or collectively by a cupule formed from numerous fused bracts, arranged individually or in small groups along an axis or at base of an androgynous inflorescence or on a separate axis. Female flower: perianth 1–7 or more; pistil 1; ovary inferior, 3–6(– 9)-loculed; style and carpels as many as locules; placentation axile; ovules 2 per locule. Fruit a nut. Seed usually solitary by abortion (but may be more than 1 in Castanea, Castanopsis, Fagus, and Formanodendron), without endosperm; embryo large. Seven to 12 genera (depending on interpretation) and 900–1000 species: worldwide except for tropical and S Africa; seven genera and 294 species (163 endemic, at least three introduced) in China. Many species are important timber trees. Nuts of Fagus, Castanea, and of most Castanopsis species are edible, and oil is extracted from nuts of Fagus. Nuts of most species of this family contain copious amounts of water soluble tannin. -
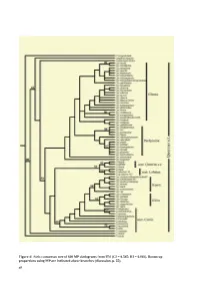
Taxonomy and Systematics of Quercus Subgenus Cyclobalanopsis
Figure 4/ Strict consensus tree of 600 MP cladograms from ITS (CI = 0.545; RI = 0.803). Bootstrap proportions using MP are indicated above branches (discussion, p. 55). 48 Taxonomy and Systematics of Quercus Subgenus Cyclobalanopsis Min Deng1, Zhekun Zhou2*, Qiansheng Li3 2. Xishuangbanna Tropical Botanical Garden, 3. School of Ecology, the Chinese Academy of Sciences, Shanghai Institute of Technology, Kunming, 650223, China. 100 Haiquan Rd., Shanghai, 201418, China 1. Chenshan Plant Sciences Research Center, the Chinese Academy of Sciences, Chenshan Botanical Garden, 3888 ChenHua Rd., Shanghai, 201602, China. Phone: +86-21- 57 79 93 82; Fax: + 86-21-67657811. [email protected]; ABSTRACT Quercus subgenus Cyclobalanopsis is one of the dominant woody plant groups in E and SE Asia, but comprehensive studies on its systematics and taxonomy are limited. In this study, we compared the leaf epidermal and acorn features of 52 species in subgenus Cyclobalanopsis and 15 species from Quercus subgenus Quercus. We also studied molecular phylogeny using DNA sequences from the nuclear ribosomal internal transcribed spacer (ITS) region and the chloroplast psbA–trnH and trnT–trnL regions. Both the leaf epidermal and acorn features indicated ʏve morphologically distinct groups in Cyclobalanopsis: 1) Gilva group (fused stellate trichomes with compound trichome base); 2) Kerrii group (with fasciculate trichomes, and radicles emerging from the basal seed scar; 3) Pachyloma group (papillae on epidermal cells, but glabrous when mature and densely yellowish woolly on the cupule walls); 4) Jenseniana group (lamellae mostly fused to the cupule wall with only the rims free); 5) Glauca group (appressed-lateral-attached trichomes).