Plumage and Behavioral Development of Nestling White Ibises
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
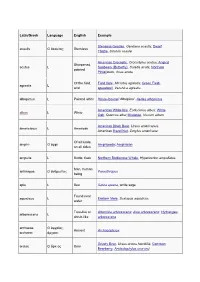
Dwarf Thistle, Cirsi
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

THE BRITISH LIST the Official List of Bird Species Recorded in Britain Sponsored by Leica Sports Optics
THE BRITISH LIST The official list of bird species recorded in Britain sponsored by Leica Sports Optics CATEGORY E SPECIES Category E comprises those species that have been recorded as introductions, human-assisted transportees or escapees from captivity, whose breeding populations (if any) are thought not to be self-sustaining. Species in Category E that have bred in the wild in Britain are designated as E*. Species listed in Category E form no part of the British List, unless they are also included in Categories A, B or C. Descriptions and definitions of Categories of the British List can be found at BOU (2017)19. Category E has been compiled largely from county/regional bird reports and other published listings, and is not exhaustive. BOURC is unable to confirm the correct identification of species in Category E, or the circumstances in which they were recorded. Doubtful cases have been excluded, pending enquiries, along with species not recorded since 1 January 1950. The BOU is responsible for maintaining the British List. Part of this responsibility is to monitor the occurrence of non-native species which may qualify for addition to Category C of the British List. To undertake this we require published information from which to work and which we can quote as reference. The BOU encourages observers to report records of non-native species to the relevant local bird recorder. Local recorders and those producing local, county or regional publications are encouraged to publish these records, and to make them available to the Rare Breeding Birds Panel (RBBP) who publish annual reports1- 7,9,10,12 of non-native species breeding in Britain in British Birds. -

Consequences of Nesting Date on Nesting Success and Juvenile Survival in White Ibis
CONSEQUENCES OF NESTING DATE ON NESTING SUCCESS AND JUVENILE SURVIVAL IN WHITE IBIS By JOHN DAVID SEMONES A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2003 Copyright 2003 by John David Semones ACKNOWLEDGMENTS This project succeeded in part due to the support and physical aid of numerous individuals and agencies. First, I would like to thank all the field technicians, volunteers, and birdwatchers across the state of Florida and those who wrote to me with roost information from Louisiana. These individuals provided invaluable aid in data collection, monitoring of roost locations, and information on lost birds. My advisor, Peter Frederick, and members of his research group, Becky Hylton and Julie Heath, provided continuous input on the project’s design and implementation, as well as support in the field. Peter, in particular, kept me pointed in the right direction and taught me the correct time and place for using the word “indubitably.” His knowledge and enthusiasm about wading birds and the Everglades are contagious and I now know more about engine mechanics than most people rightfully should know. Throughout this process my committee members, Ken Meyer and Madan Oli, provided feedback on my ideas and helped make this a well-rounded project. Ken’s advice on telemetry flights and radio- tracking methods was particularly instrumental. I am grateful to A.R.M. Loxahatchee National Wildlife Refuge, Everglades National Park, and the Palm Beach County Waste Facility for permission to conduct this research with unfettered access to these locations. -

Internest Displacement of White Ibis Eggs
SHORT COMMUNICATIONS 273 of the Fitzpatrick Institutes’ 25th Anniversary Expedition to Chile. Financial support was received from the South African CSIR and the University of Cape Town. LITERATURE CITED CHAPMAN, S. E. 1973. The grey gull, Lams modestus.Sea Swallow 22:7-10. DUFFY, D. C. 1980. Patterns of piracy by Peruvian seabirds: a depth hypothesis. Ibis 122: 521-525. -. 1983. The foraging ecology of Peruvian seabirds. Auk 100:800-810. HARRISON, P. 1983. Seabirds: an identification guide. Croom Helm, Beckenham, England. HOWELL, T. R., B. ARAYA, AND W. R. MILLIE. 1974. Breeding biology of the gray gull Lams modestus.Univ. Calif. Publ. Zool. 104:1-57. JOHNSON,A. W. 1965. Birds of Chile. Vol. II. Platt Estab. Graficos, Buenos Aires, Argen- tina. MURPHY, R. C. 1936. Oceanic birds of South America. Macmillan, New York, New York. SEARCY,W. A. 1978. Foraging successin three age classes of Glaucous-winged Gulls. Auk 95:586-590. VERBEEK, N. A. M. 1977. Comparative feeding ecology of adult and immature Herring Gulls. Wilson Bull. 89:4 15-42 1. P. G. RYAN, P. A. R. HOCKEY, AND A. L. BOSMAN, Fitzpatrick Inst., Univ. Cape Town, Rondebosch7700, South Africa. ReceivedI7 June 1986. accepted6 Oct. 1986. WilsonBull., 99(2), 1987, pp. 273-275 Internest displacement of White Ibis eggs.-During a study of nesting success at a White Ibis (Eudocimusa/bus) colony (Shields and Pamell 1986), I observed five cases in which an egg laid in one nest was subsequently found in another nest in the same tree or shrub. The study was conducted during the 1983 and 1984 breeding seasons at Battery Island, North Carolina (33”54N,’ 78”olW),’ where White Ibises nested in a maritime shrub thicket (see Shields and Pamell 1986 for a complete description of the area). -

Population Energetics of the American White Ibis
POPULATION ENERGETICS OF THE AMERICAN WHITE IBIS JAMES A. KUSHLAN ABSTRACT.--TheWhite Ibis populationnesting in the fluctuating water ecosystemof southern Florida numbered up to 62,000 birds and used several nesting habitats including islands in large lakes, the vast freshwater marshesof the Everglades, and coastal estuaries. Periodic reproduction Downloaded from https://academic.oup.com/auk/article/94/1/114/5209013 by guest on 25 September 2021 at large inland colonies,accounting for 93-98% of the nesting birds, sustainsthe population at its current level, but smaller, more consistantlysuccessful coastal colonies provide recruitmentduring years of inland nesting failure. Clutch size, brood reduction pattern, and energy requirements showed intraregional variation. Clutch size differences may be related to habitat, with lowered clutch sizes along the coast. Despite clutch size differences,the number of young fledged was similar in different colonies. IntraregionaI variation in timing of brood reduction resulted in differ- encesin the energyrequired for nesting.The daily energyexpenditure of White Ibis was estimated from the time-activity budget and multiples of measured metabolic rates. Although existence metabolismwas similar to that predictedby Kendeigh's(1970) regression,daily energyexpenditure was considerably below that predicted by extrapolating King's (1974) regression, suggestinga slower increaseof DEE at higher body weights. The White Ibis consumedabout 21% of its body weightdaily, similar to that found in otherstudies of ciconiiformes.The energyrequired to raisea broodof young was 9.2 9.9 x 103kcal. The nestingpopulation expended 930 and 640 x 106kcal during 1972 and 1973 respectively. Over 70% of this energy was derived from the Everglades and over 90• from inland habitats. -

Frederick, P. C. 1987. Chronic Tidally-Induced Nest Failure in A
Cooper Ornithological Society Chronic Tidally-Induced Nest Failure in a Colony of White Ibises Author(s): Peter Frederick Source: The Condor, Vol. 89, No. 2 (May, 1987), pp. 413-419 Published by: University of California Press on behalf of the Cooper Ornithological Society Stable URL: http://www.jstor.org/stable/1368495 Accessed: 06/10/2009 16:04 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=ucal. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. University of California Press and Cooper Ornithological Society are collaborating with JSTOR to digitize, preserve and extend access to The Condor. -

2008 - 2015 Wildlife Strike Analyses (Ibis)
International Civil Aviation Organization ELECTRONIC BULLETIN For information only EB 2017/25 12 May 2017 2008 - 2015 WILDLIFE STRIKE ANALYSES (IBIS) The analyses of wildlife strike reports for the years 2008 to 2015 are based on 97 751 reports, received from ninety-one States on strikes occurring in 105 States and territories as shown at Attachment A. A summary of wildlife strikes reported to the ICAO Bird Strike Information System (IBIS) for the years 2008 to 2015 is included at Attachment B, IBIS World Wildlife Strike Statistics at Attachment C and a list of wildlife types at Attachment D. The above attachments (available in English only) can be found at www.icao.int/IBIS. The analyses of wildlife strike data and observing and monitoring of wildlife activities can reveal trends that will assist airport authorities in identifying areas of concern, which should be addressed through a well-managed wildlife control programme. Wildlife strike statistics can also be analysed to determine those times of year or day when wildlife control is needed the most. In order to better facilitate occurrence reporting and data analysis, ICAO now has replaced the old IBIS computer-application with a new reporting system based on the European Co-ordination Centre for Accident and Incident Reporting Systems (ECCAIRS) platform. A User Manual and Software Installation Manual can be downloaded at www.icao.int/IBIS. States are encouraged to submit wildlife strike reports either via ECCAIRS e5f/e4f files, or via an ECCAIRS Excel-based form that can also be downloaded at www.icao.int/IBIS. Enclosures: A — List of States and Territories for the years 2008 - 2015 B — Summary of Wildlife Strikes reported to ICAO Bird Strike Information System (IBIS) for the years 2008 - 2015 C — IBIS World Wildlife Strike Statistics 2008 - 2015 D — List of wildlife types for the years 2008 - 2015 Issued under the authority of the Secretary General 999 Robert-Bourassa Boulevard Tel.: +1 514-954-8219 ext. -

Population Ecology of the Australian White Ibis, Threskiornis Molucca, In
University of Technology, Sydney Faculty of Science Department of Environmental Sciences Population ecology of the Australian White Ibis, Threskiornis molucca, in the urban environment. Andrew Charles Michael Smith BSc (University of Technology, Sydney) 2009 Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Technology, Sydney Faculty of Science Department of Environmental Sciences PhD Thesis Population ecology of the Australian White Ibis, Threskiornis molucca, in the urban environment. Andrew Charles Michael Smith 2009 Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy i Abstract The Australian White Ibis (Threskiornis molucca) has dramatically increased in many coastal urban environments, while it has decreased in large areas of its traditional environment range in inland Australia since the 1970s. Ibis are often viewed as pests in urban environments due to the social, economical and environmental problems they can cause. Current, management of ibis in the urban environment predominately focuses on restricting their breeding success, in order to reduce abundances. Management can be costly, labour intensive and limited in its success, due to a lack of detailed knowledge of the ecology of urban ibis. The focus of this thesis is to explore various ecological parameters of urban ibis to increase the effectiveness of their management. Three major breeding/roosting colonies of ibis were monitored weekly for a whole year (2005 to 2006). In addition, five major landfills for domestic waste were investigated for avian abundances and diversity. My main aims were to provide details on the reproductive biology, population dynamics, local and regional movements and the use of landfills by ibis. -

Population Energetics of the American White Ibis
POPULATION ENERGETICS OF THE AMERICAN WHITE IBIS JAMES A. KUSHLAN ABSTRACT.-The White Ibis population nesting in the fluctuating water ecosystem of southern Florida numbered up to 62,000 birds and used several nesting habitats including islands in large lakes, the vast freshwater marshes of the Everglades, and coastal estuaries. Periodic reproduction at large inland colonies, accounting for 93-98% of the nesting birds, sustains the population at its current level, but smaller, more consistantly successful coastal colonies provide recruitment during years of inland nesting failure. Clutch size, brood reduction pattern, and energy requirements showed intraregional variation. Clutch size differences may be related to habitat, with lowered clutch sizes along the coast. Despite clutch size differences, the number of young fledged was similar in different colonies. Intraregional variation in timing of brood reduction resulted in differ- ences in the energy required for nesting. The daily energy expenditure of White Ibis was estimated from the time-activity budget and multiples of measured metabolic rates. Although existence metabolism was similar to that predicted bv Kendeigh's (1970) regression, daily energy expenditure was considerably below that predicted by extrapolating King's (1974) regression, suggesting a slower increase of DEE at higher body weights. The White Ibis consumed about 21% of its body weight daily, similar to that found in other studies of ciconiiformes. The energy required to raise a brood of young was 9.2-9.9 x io' kcal. The nesting population expended 930 and 640 x 106 kcal during 1972 and 1973 respectively. Over 70% of this energy was derived from the Everglades and over 90% from inland habitats. -

Ornithological Literature
Wilson Bull., 107(l), 1995, pp. 185-191 ORNITHOLOGICAL LITERATURE RESTLESSENERGY-A BIOGRAPHYOF WILLIAM ROWAN. By Marianne G. Ainley, VChicule Press, Montreal, Canada. 1993:368 pp., colored cover, 22 illustrations including black-and- white photographs, manuscript pages, and art work. $19.95.-Over the years when I lectured in ecology and ornithology classes about the effect of light on birds, I would mention “so- meones”’ experiments exposing juncos in Canada to increasing lengths of artificial daylight in autumn to see if the birds would fly north. Both I-and presumably the students-viewed this as (1) a clever endeavor and (2) worthy of a chuckle. But did some scientist really try this? Or was it only apocryphal? In the pages of “Restless Energy” came the revelation, not only about this but many other of my lecture topics, as the life work of the remarkable European-born Canadian biologist William Rowan unfolded. The lad of ten (in 1901) who carried, in a tea infusion ball, “migrating French flies to England” would proceed, over his lifetime (he died in 1957), to do classical studies on the mechanism of migration, the effect of light duration on juncos, crows, and other species, light and seasonal reproduction in animals, the role of hormones in bird behavior, wildlife population cycles (it was Rowan who originally proposed the famous snowshoe hare/lynx research), and ecological studies of waterfowl and other vertebrates. Biographer Ainley was aware of these accomplishments through her work on a history of Canadian ornithology. But was -

Dietary Niche Relationships of White Ibis, Tricolored Heron And
DIETARY NICHE RELATIONSHIPS OF WHITE IBIS, TRICOLORED HERON AND SNOWY EGRET NESTLINGS IN THE NORTHERN EVERGLADES by Robin A. Boyle A Thesis Submitted to the Faculty of The Charles E. Schmidt College of Science in Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, Florida December, 2010 ACKNOWLEDGEMENTS The South Florida Management District – Everglades Division provided funding for this project. I especially want to thank the district biologists, Dr. Mark Cook and Mac Kobza, who played a fundamental role in the collection of boluses and provided access to wading bird colonies. I also want to thank Brent Bellinger and James Beerens who also helped me in the field. My research and this manuscript would not have been possible without the help of several people. First, I want to thank my advisor, Dr. Nathan Dorn, for his continuous support, guidance and faith in me. His willingness to take me on as a graduate student has provided me with the foundations to become an ecologist. I also want to thank my thesis committee members, Dr. Dale Gawlik and Dr. Erik Noonburg, who provided comments and helpful suggestions on earlier versions of this manuscript. My fellow lab members, Christopher Kellogg, Craig van der Heiden, and Jacob Bransky, patiently listened to and provided comments on thesis drafts and presentations. Finally, I would like to thank my parents, Michael and Linda Boyle, who passed down their love of nature, encouraged me to become whatever I wanted to be, and provided unconditional love and support in all my endeavors. -

Genetic Diversity and Host Specificity of Blood Parasites of Wading
GENETIC DIVERSITY AND HOST SPECIFICITY OF BLOOD PARASITES OF WADING BIRDS IN SOUTHERN FLORIDA by SARAH M. COKER (Under the Direction of Michael J. Yabsley) ABSTRACT White ibis (Eudocimus albus) have become increasingly urbanized, many now relying heavily on urban/suburban habitats. Avian haemosporidia can cause acute disease and reduced fitness. We hypothesized that prevalence of infection differs by site and has high genetic parasite diversity, with some parasites more host species specific than others. Blood from white ibis from Palm Beach, Lee, and Broward Counties in Florida were tested for haemoparasites by analyzing Giemsa-stained thin blood smears and PCR. In Palm Beach, Lee, and Broward Counties, 68%, 61%, and 27% were positive, respectively. Sequences of 139 positives revealed a novel haplotype of Haemoproteus (hWHIB01). Morphologically, parasites were identified as H. plataleae. Parasitemias of 66 positive birds were very low (average 0.085%, range <0.001%- 0.890%). An infection with hWHIB01 was detected in a green heron (Butorides virescens). No Plasmodium infections were detected in white ibis, despite sympatric Pelicaniformes from Lee and Broward Counties with Haemoproteus and Plasmodium infections. INDEX WORDS: Avian malaria, Hematozoa, White ibis, Eudocimus albus, Pelicaniformes, Haemoproteus plataleae GENETIC DIVERSITY AND HOST SPECIFICITY OF BLOOD PARASITES OF WADING BIRDS IN SOUTHERN FLORIDA by SARAH M. COKER B.S., Mary Baldwin College, 2012 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2015 © 2015 Sarah M. Coker All Rights Reserved GENETIC DIVERSITY AND HOST SPECIFICITY OF BLOOD PARASITES OF WADING BIRDS IN SOUTHERN FLORIDA by SARAH M.