Frederick, P. C. 1987. Chronic Tidally-Induced Nest Failure in A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

January 2020.Indd
BROWN PELICAN Photo by Rob Swindell at Melbourne, Florida JANUARY 2020 Editors: Jim Jablonski, Marty Ackermann, Tammy Martin, Cathy Priebe Webmistress: Arlene Lengyel January 2020 Program Tuesday, January 7, 2020, 7 p.m. Carlisle Reservation Visitor Center Gulls 101 Chuck Slusarczyk, Jr. "I'm happy to be presenting my program Gulls 101 to the good people of Black River Audubon. Gulls are notoriously difficult to identify, but I hope to at least get you looking at them a little closer. Even though I know a bit about them, I'm far from an expert in the field and there is always more to learn. The challenge is to know the particular field marks that are most important, and familiarization with the many plumage cycles helps a lot too. No one will come out of this presentation an expert, but I hope that I can at least give you an idea what to look for. At the very least, I hope you enjoy the photos. Looking forward to seeing everyone there!” Chuck Slusarczyk is an avid member of the Ohio birding community, and his efforts to assist and educate novice birders via social media are well known, yet he is the first to admit that one never stops learning. He has presented a number of programs to Black River Audubon, always drawing a large, appreciative gathering. 2019 Wellington Area Christmas Bird Count The Wellington-area CBC will take place Saturday, December 28, 2019. Meet at the McDonald’s on Rt. 58 at 8:00 a.m. The leader is Paul Sherwood. -
Dwarf Thistle, Cirsi
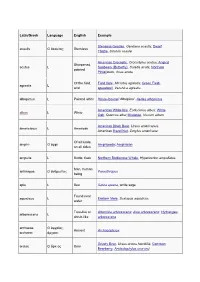
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Developing a Research Network on Glossy Ibis, a Neglected Cosmopolitan Species
Developing a research network on Glossy ibis, a neglected cosmopolitan species Álvaro Arenas Patiño © 2014 The Project in a nutshell The Glossy ibis (Plegadis falcinellus) is the only cosmopolitan species of the Threskiornithidae family (ibis and spoonbills) and among the most widely distributed bird species in the world ([1] see map at the bottom of the document). Nonetheless, no knowledge exists on the intraspecific phylogeny and very little is known about the (meta)populations dynamics. We aim to fill this gap by setting up a research network and by implementing modern analytical tools (e.g. molecular and statistical) we nowadays dispose of. Some puzzling aspects of Glossy ibis abundance and distribution Little is known about the movement ecology of this species especially when focusing on a large spatial scale. In most ornithology books the Glossy ibis is described as migratory with nomadic elements [2] with populations at tropics being more sedentary than others. However, references are rarely provided and, to the best of our knowledge, only a few data have been ever collected on the Glossy ibis dispersal behavior. According to historical records, breeding was very rare in W Europe while common in North Africa and S Spain before the 20th century when it became almost absent in all these regions [3–5]. In 1996 seven pairs settled at Doñana, a wetland area in SW Spain, from where the species begun an astonishing population increase reaching about 8,000 pairs in current times [6,7]. Since then, the Glossy ibis has started to colonize other areas in W Europe and the Mediterranean Basin from where it had been absent for decades or even had never been recorded as a breeder species. -

New Observations of the Andean Ibis (Theristicus Branickii
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Fall 12-1-2014 New Observations of the Andean Ibis (Theristicus branickii, Threskiornithidae): Distribution, Movements, and Behavior Near Volcán Antisana Benjamin West SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Latin American Studies Commons, Other Ecology and Evolutionary Biology Commons, Population Biology Commons, and the Zoology Commons Recommended Citation West, Benjamin, "New Observations of the Andean Ibis (Theristicus branickii, Threskiornithidae): Distribution, Movements, and Behavior Near Volcán Antisana" (2014). Independent Study Project (ISP) Collection. 2019. https://digitalcollections.sit.edu/isp_collection/2019 This Article is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. New Observations of the Andean Ibis (Theristicus branickii , Threskiornithidae): Distribution, Movements, and Behavior Near Volcán Antisana West, Benjamin M. Academic Directors: Silva, Xavier and Robayo, Javier Project Advisor: Williamson, Jessie Bowdoin College Biology South America, Ecuador, Napo Province, Reserva Ecológica Antisana Submitted in partial fulfillment of the requirements for Ecuador: Comparative Ecology and Conservation, SIT Study Abroad, Fall 2014 SIT Ecuador: Ecology, Fall 2014 West Abstract The Andean Ibis (Theristicus branickii ) of the highland grasslands of Ecuador, Peru, and Bolivia is listed globally as Near Threatened and Critically Endangered in Ecuador. The Ecuadorian population is estimated at 100 individuals and is restricted to the vicinities of Volcán Antisana and Volcán Cotopaxi. -

Plumage and Behavioral Development of Nestling White Ibises
Wilson Bull., 102(2), 1990, pp. 226-238 PLUMAGE AND BEHAVIORAL DEVELOPMENT OF NESTLING WHITE IBISES TONI L. DE SANTO,’ SUSAN G. MCDOWELL,~ AND KEITH L. BILDSTEIN~ Ans-rticr.- We describe the physical characteristicsand behavioral development OI 17 hand-reared and more than 400 parent-reared nestling White Ibises (Eudocimus albus) hatched in 1985 through 1988 at Pumpkinseed Island, a large colony site in coastal South Carolina. Hatchling ibises are covered with a Pale Neutral Gray to Jet Black natal plumage. About 30% of the hatchlingspossess a tuft of white feathers on their crown, and this pattern persiststhroughout the nestling period. Juvenal plumage, which is complete by 60 days, is mainly Vandyke Brown and Blackish Neutral Gray dorsally and creamy white ventrally. The bill, which is straight at hatching, begins to curve downward at about 14 days. Nestling White Ibises exhibit considerableindividual variation in bill markings from approximately 10 days of age through fledging. Increasingly persistent beggingvocalizations begin within hours of hatching. Nestlings walk on partially extended legsat eight days of age, pirate food from other nestlingsand form crechesat 2 1 days of age, and fledge and join all juvenile and mixed-age feeding flocks at 45-55 days of age. We suggestthat the phenotypic variability in plumage, bill coloration, and beggingcalls we describeenables parental ibises to identify more easily their offspringat the colony site. Received27 Feb. 1989, accepted12 Nov. 1989. Although the plumage and behavioral development of several species of wading birds has been studied in considerable detail (e.g., Hammerkops [&opus umbretta], Wilson et al. 1988; storks, Kahl 1962, 1966; Thomas 1984; herons, Gross 1923; Gavin0 and Dickerman 1972; Juarez and Dickerman 1972; Mc Vaugh 1972,1975; Snow 1974; Merritt 198 l), there are few detailed studies of juvenile ibises. -

Checklist of Amphibians, Reptiles, Birds and Mammals of New York
CHECKLIST OF AMPHIBIANS, REPTILES, BIRDS AND MAMMALS OF NEW YORK STATE Including Their Legal Status Eastern Milk Snake Moose Blue-spotted Salamander Common Loon New York State Artwork by Jean Gawalt Department of Environmental Conservation Division of Fish and Wildlife Page 1 of 30 February 2019 New York State Department of Environmental Conservation Division of Fish and Wildlife Wildlife Diversity Group 625 Broadway Albany, New York 12233-4754 This web version is based upon an original hard copy version of Checklist of the Amphibians, Reptiles, Birds and Mammals of New York, Including Their Protective Status which was first published in 1985 and revised and reprinted in 1987. This version has had substantial revision in content and form. First printing - 1985 Second printing (rev.) - 1987 Third revision - 2001 Fourth revision - 2003 Fifth revision - 2005 Sixth revision - December 2005 Seventh revision - November 2006 Eighth revision - September 2007 Ninth revision - April 2010 Tenth revision – February 2019 Page 2 of 30 Introduction The following list of amphibians (34 species), reptiles (38), birds (474) and mammals (93) indicates those vertebrate species believed to be part of the fauna of New York and the present legal status of these species in New York State. Common and scientific nomenclature is as according to: Crother (2008) for amphibians and reptiles; the American Ornithologists' Union (1983 and 2009) for birds; and Wilson and Reeder (2005) for mammals. Expected occurrence in New York State is based on: Conant and Collins (1991) for amphibians and reptiles; Levine (1998) and the New York State Ornithological Association (2009) for birds; and New York State Museum records for terrestrial mammals. -

THE BRITISH LIST the Official List of Bird Species Recorded in Britain Sponsored by Leica Sports Optics
THE BRITISH LIST The official list of bird species recorded in Britain sponsored by Leica Sports Optics CATEGORY E SPECIES Category E comprises those species that have been recorded as introductions, human-assisted transportees or escapees from captivity, whose breeding populations (if any) are thought not to be self-sustaining. Species in Category E that have bred in the wild in Britain are designated as E*. Species listed in Category E form no part of the British List, unless they are also included in Categories A, B or C. Descriptions and definitions of Categories of the British List can be found at BOU (2017)19. Category E has been compiled largely from county/regional bird reports and other published listings, and is not exhaustive. BOURC is unable to confirm the correct identification of species in Category E, or the circumstances in which they were recorded. Doubtful cases have been excluded, pending enquiries, along with species not recorded since 1 January 1950. The BOU is responsible for maintaining the British List. Part of this responsibility is to monitor the occurrence of non-native species which may qualify for addition to Category C of the British List. To undertake this we require published information from which to work and which we can quote as reference. The BOU encourages observers to report records of non-native species to the relevant local bird recorder. Local recorders and those producing local, county or regional publications are encouraged to publish these records, and to make them available to the Rare Breeding Birds Panel (RBBP) who publish annual reports1- 7,9,10,12 of non-native species breeding in Britain in British Birds. -

Sacred Ibis (Threskiornis Aethiopicus)1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. WEC267 Florida's Introduced Birds: Sacred Ibis (Threskiornis aethiopicus)1 Steve Johnson and Monica McGarrity2 Many non-native birds have been introduced in prey. Sacred Ibises are larger than Florida's native Florida—perhaps as many as 200 species! Of these, ibises. Adults are approximately 30 inches long (75 at least 16 introduced species are considered cm) with a wingspan of 44–49 inches (112–124 established, according to various authorities, and cm) and weigh about 3 pounds (1.4 kg). Sacred Ibises some are now considered invasive and could have (Fig. 1) have mostly white bodies and wings; the serious impacts in Florida. This fact sheet introduces trailing edges of their wings (tips of the feathers) are the Sacred Ibis, and is one of a series of fact sheets gray-black. They have very distinctive long, black about Florida's established non-native birds and their feathers or plumes on their rumps (Fig. 1). During the impacts on our native ecosystems, economy, and the breeding season the feathers on the sides of their quality of life of Floridians. For more information on chests and on the outer wings (near the edge when Florida's introduced birds, how they got here, and the folded) may have a yellowish (or reddish) tinge, and problems they cause, read "Florida's Introduced their lower legs may be tinged with reddish-copper; Birds: An Overview" bare patches of scarlet-red skin may also be visible (http://edis.ifas.ufl.edu/UW297) and the other fact under their wings. -

Consequences of Nesting Date on Nesting Success and Juvenile Survival in White Ibis
CONSEQUENCES OF NESTING DATE ON NESTING SUCCESS AND JUVENILE SURVIVAL IN WHITE IBIS By JOHN DAVID SEMONES A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2003 Copyright 2003 by John David Semones ACKNOWLEDGMENTS This project succeeded in part due to the support and physical aid of numerous individuals and agencies. First, I would like to thank all the field technicians, volunteers, and birdwatchers across the state of Florida and those who wrote to me with roost information from Louisiana. These individuals provided invaluable aid in data collection, monitoring of roost locations, and information on lost birds. My advisor, Peter Frederick, and members of his research group, Becky Hylton and Julie Heath, provided continuous input on the project’s design and implementation, as well as support in the field. Peter, in particular, kept me pointed in the right direction and taught me the correct time and place for using the word “indubitably.” His knowledge and enthusiasm about wading birds and the Everglades are contagious and I now know more about engine mechanics than most people rightfully should know. Throughout this process my committee members, Ken Meyer and Madan Oli, provided feedback on my ideas and helped make this a well-rounded project. Ken’s advice on telemetry flights and radio- tracking methods was particularly instrumental. I am grateful to A.R.M. Loxahatchee National Wildlife Refuge, Everglades National Park, and the Palm Beach County Waste Facility for permission to conduct this research with unfettered access to these locations. -

Trapping White Ibises with Rocket Nets and Mist Nets in the Florida Everglades
J. Field Ornithol. 74(2):187±192, 2003 Trapping White Ibises with rocket nets and mist nets in the Florida Everglades Julie A. Heath1 and Peter C. Frederick Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, Florida 32611 USA Received 30 April 2002; accepted 19 September 2002 ABSTRACT. We lured White Ibises (Eudocimus albus) to trap sites with decoy plastic ¯amingos and captured them with a rocket net or mist nets. Our ability to attract ibises to a site (and consequently our capture success) was affected by vegetation height and water depth but not by decoy numbers or their arrangement. Both the rocket net (37 ibises) and mist nets (97 ibises) caught birds. The number of birds captured per day was the same for both methods, but the rocket net trap captured more birds per set than did mist nets. Because mist nets were easy to reset we captured 1±2 birds per set multiple times in one day with mist nets. We preferred mist nets over rocket net traps because mist nets allowed for shorter bird processing times, greater ease of set up, and fewer safety considerations for transport and operation of the trap. Also, rocket nets required more equipment and expense. Although we discouraged other species from landing at a trap site, there was evidence that these techniques may also be useful for capturing other wading birds. SINOPSIS. Captura de ciguenÄas (Eudocimus albus) con redes de cohetes y redes de niebla en la Florida Atraimos ciguenÄas (Eudocimus albus) a lugares particulares utilizando senÄuelos de ¯amencos para atraparlas con redes de cohetes y con redes de niebla. -

Observations on the Behaviour of the Scarlet Ibis, Eudocimus Ruber, In
Bijdragen tot de Dierkunde, 55 (2): 219-232 — 1985 Observations on the behaviour of the Scarlet Ibis, Eudocimus ruber, in Artis Zoo, Amsterdam by H. Albrecht R.E. Spil M.W. van Walstijn & Royal Zoological Society "Natura Artis Magistra", Plantage Kerklaan 38, 1018 CZ Amsterdam, The Netherlands & Department of Animal Behaviour, University of Amsterdam, The Netherlands Abstract without overt signs of aggression. The hierarchy was not linear but could be subdivided into three groupings. The The Scarlet Ibis, Eudocimus ruber (Linnaeus), fam. of bird correlated with status a was sex-dependent, weight Threskiornithidae, is the closest relative ofthe White Ibis, and of but length the bill, not with nest-ownership or age. Eudocimus albus (Linnaeus). The two species live in adjoin- The breeding behaviour of the Scarlet Ibis and the is known about the ing geographical areas. Nothing White Ibis seems to be so similar that, bearing in mind Scarlet Ibis’s in the wild and little breeding biology only the be their similarity in morphology, question may posed about its breeding biology in captivity. A better under- whether they are conspecific. standing of the breeding of this species in captivity should its and also elucidate its help to improve management to Zusammenfassung relationship with its much better known counterpart, the White Ibis. Der Rote Sichler, Eudocimus ruber (Linnaeus), Fam. Three of behaviour in types display are recognized Threskiornithidae, ist der vikariierende nächste Verwand- the the and courtship: snap display, dipping snap display te des Weißen Sichlers, Eudocimus albus (Linnaeus). Von also in The latter occurs greeting ceremonies, twig pulling. -

Internest Displacement of White Ibis Eggs
SHORT COMMUNICATIONS 273 of the Fitzpatrick Institutes’ 25th Anniversary Expedition to Chile. Financial support was received from the South African CSIR and the University of Cape Town. LITERATURE CITED CHAPMAN, S. E. 1973. The grey gull, Lams modestus.Sea Swallow 22:7-10. DUFFY, D. C. 1980. Patterns of piracy by Peruvian seabirds: a depth hypothesis. Ibis 122: 521-525. -. 1983. The foraging ecology of Peruvian seabirds. Auk 100:800-810. HARRISON, P. 1983. Seabirds: an identification guide. Croom Helm, Beckenham, England. HOWELL, T. R., B. ARAYA, AND W. R. MILLIE. 1974. Breeding biology of the gray gull Lams modestus.Univ. Calif. Publ. Zool. 104:1-57. JOHNSON,A. W. 1965. Birds of Chile. Vol. II. Platt Estab. Graficos, Buenos Aires, Argen- tina. MURPHY, R. C. 1936. Oceanic birds of South America. Macmillan, New York, New York. SEARCY,W. A. 1978. Foraging successin three age classes of Glaucous-winged Gulls. Auk 95:586-590. VERBEEK, N. A. M. 1977. Comparative feeding ecology of adult and immature Herring Gulls. Wilson Bull. 89:4 15-42 1. P. G. RYAN, P. A. R. HOCKEY, AND A. L. BOSMAN, Fitzpatrick Inst., Univ. Cape Town, Rondebosch7700, South Africa. ReceivedI7 June 1986. accepted6 Oct. 1986. WilsonBull., 99(2), 1987, pp. 273-275 Internest displacement of White Ibis eggs.-During a study of nesting success at a White Ibis (Eudocimusa/bus) colony (Shields and Pamell 1986), I observed five cases in which an egg laid in one nest was subsequently found in another nest in the same tree or shrub. The study was conducted during the 1983 and 1984 breeding seasons at Battery Island, North Carolina (33”54N,’ 78”olW),’ where White Ibises nested in a maritime shrub thicket (see Shields and Pamell 1986 for a complete description of the area).