Avian Visual Perception: Interocular and Intraocular Transfer and Head
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shahezan Issani Report Environment and Social Impact Assessment for Road Asset 2020-03-02
Draft Initial Environmental Examination Project Number: 53376-001 September 2020 IND: DBL Highway Project Prepared by AECOM India Private Limited The initial environmental examination is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “Terms of Use” section of this website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. FINAL ESIA Environment and Social Impact Assessment (ESIA) of Road Asset Anandapuram-Pendurthi-Anakapalli Section of NH-16 Dilip Buildcon Limited September 19, 2020 Environment and Social Impact Assessment of Road Asset – Anandapuram – Pendurthi – Ankapalli Section of NH 16, India FINAL Quality information Prepared by Checked by Verified by Approved by Shahezan Issani Bhupesh Mohapatra Bhupesh Mohapatra Chetan Zaveri Amruta Dhamorikar Deepti Bapat Revision History Revision Revision date Details Authorized Name Position 01 23 April 2020 First cut ESIA report without Yes Chetan Zaveri Executive Director monitoring data 02 30 April 2020 Draft ESIA report without monitoring Yes Chetan Zaveri Executive Director data 03 9 July 2020 Final ESIA report with monitoring Yes Chetan Zaveri Executive Director data and air modelling -

Current Perspectives on the Evolution of Birds
Contributions to Zoology, 77 (2) 109-116 (2008) Current perspectives on the evolution of birds Per G.P. Ericson Department of Vertebrate Zoology, Swedish Museum of Natural History, P.O. Box 50007, SE-10405 Stockholm, Sweden, [email protected] Key words: Aves, phylogeny, systematics, fossils, DNA, genetics, biogeography Contents (cf. Göhlich and Chiappe, 2006), making feathers a plesiomorphy in birds. Indeed, only three synapo- Systematic relationships ........................................................ 109 morphies have been proposed for Aves (Chiappe, Genome characteristics ......................................................... 111 2002), although monophyly is never seriously ques- A comparison with previous classifications ...................... 112 Character evolution ............................................................... 113 tioned: 1) the caudal margin of naris nearly reaching Evolutionary trends ............................................................... 113 or overlapping the rostral border of the antorbital Biogeography and biodiversity ............................................ 113 fossa (in the primitive condition the caudal margin Differentiation and speciation ............................................. 114 of naris is farther rostral than the rostral border of Acknowledgements ................................................................ 115 the antorbital fossa), 2) scapula with a prominent References ................................................................................ 115 acromion, -

Whistler3 Frontcover
The Whistler is the occasionally issued journal of the Hunter Bird Observers Club Inc. ISSN 1835-7385 The aims of the Hunter Bird Observers Club (HBOC), which is affiliated with Bird Observation and Conservation Australia, are: To encourage and further the study and conservation of Australian birds and their habitat To encourage bird observing as a leisure-time activity HBOC is administered by a Committee: Executive: Committee Members: President: Paul Baird Craig Anderson Vice-President: Grant Brosie Liz Crawford Secretary: Tom Clarke Ann Lindsey Treasurer: Rowley Smith Robert McDonald Ian Martin Mick Roderick Publication of The Whistler is supported by a Sub-committee: Mike Newman (Joint Editor) Harold Tarrant (Joint Editor) Liz Crawford (Production Manager) Chris Herbert (Cover design) Liz Huxtable Ann Lindsey Jenny Powers Mick Roderick Alan Stuart Authors wishing to submit manuscripts for consideration for publication should consult Instructions for Authors on page 61 and submit to the Editors: Mike Newman [email protected] and/or Harold Tarrant [email protected] Authors wishing to contribute articles of general bird and birdwatching news to the club newsletter, which has 6 issues per year, should submit to the Newsletter Editor: Liz Crawford [email protected] © Hunter Bird Observers Club Inc. PO Box 24 New Lambton NSW 2305 Website: www.hboc.org.au Front cover: Australian Painted Snipe Rostratula australis – Photo: Ann Lindsey Back cover: Pacific Golden Plover Pluvialis fulva - Photo: Chris Herbert The Whistler is proudly supported by the Hunter-Central Rivers Catchment Management Authority Editorial The Whistler 3 (2009): i-ii The Whistler – Editorial The Editors are pleased to provide our members hopefully make good reading now, but will and other ornithological enthusiasts with the third certainly provide a useful point of reference for issue of the club’s emerging journal. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Appendix 1: Maps and Plans Appendix184 Map 1: Conservation Categories for the Nominated Property
Appendix 1: Maps and Plans Appendix184 Map 1: Conservation Categories for the Nominated Property. Los Alerces National Park, Argentina 185 Map 2: Andean-North Patagonian Biosphere Reserve: Context for the Nominated Proprty. Los Alerces National Park, Argentina 186 Map 3: Vegetation of the Valdivian Ecoregion 187 Map 4: Vegetation Communities in Los Alerces National Park 188 Map 5: Strict Nature and Wildlife Reserve 189 Map 6: Usage Zoning, Los Alerces National Park 190 Map 7: Human Settlements and Infrastructure 191 Appendix 2: Species Lists Ap9n192 Appendix 2.1 List of Plant Species Recorded at PNLA 193 Appendix 2.2: List of Animal Species: Mammals 212 Appendix 2.3: List of Animal Species: Birds 214 Appendix 2.4: List of Animal Species: Reptiles 219 Appendix 2.5: List of Animal Species: Amphibians 220 Appendix 2.6: List of Animal Species: Fish 221 Appendix 2.7: List of Animal Species and Threat Status 222 Appendix 3: Law No. 19,292 Append228 Appendix 4: PNLA Management Plan Approval and Contents Appendi242 Appendix 5: Participative Process for Writing the Nomination Form Appendi252 Synthesis 252 Management Plan UpdateWorkshop 253 Annex A: Interview Guide 256 Annex B: Meetings and Interviews Held 257 Annex C: Self-Administered Survey 261 Annex D: ExternalWorkshop Participants 262 Annex E: Promotional Leaflet 264 Annex F: Interview Results Summary 267 Annex G: Survey Results Summary 272 Annex H: Esquel Declaration of Interest 274 Annex I: Trevelin Declaration of Interest 276 Annex J: Chubut Tourism Secretariat Declaration of Interest 278 -

Insular Vertebrate Evolution: the Palaeontological Approach': Monografies De La Societat D'història Natural De Les Balears, Lz: Z05-118
19 DESCRIPTION OF THE SKULL OF THE GENUS SYLVIORNIS POPLIN, 1980 (AVES, GALLIFORMES, SYLVIORNITHIDAE NEW FAMILY), A GIANT EXTINCT BIRD FROM THE HOLOCENE OF NEW CALEDONIA Cécile MOURER-CHAUVIRÉ & Jean Christophe BALOUET MOUREH-CHAUVIRÉ, C. & BALOUET, l.C, zoos. Description of the skull of the genus Syluiornis Poplin, 1980 (Aves, Galliformes, Sylviornithidae new family), a giant extinct bird from the Holocene of New Caledonia. In ALCOVER, J.A. & BaVER, P. (eds.): Proceedings of the International Symposium "Insular Vertebrate Evolution: the Palaeontological Approach': Monografies de la Societat d'Història Natural de les Balears, lZ: Z05-118. Resum El crani de Sylviornismostra una articulació craniarostral completament mòbil, amb dos còndils articulars situats sobre el rostrum, el qual s'insereix al crani en dues superfícies articulars allargades. La presència de dos procesos rostropterigoideus sobre el basisfenoide del rostrum i la forma dels palatins permet confirmar que aquest gènere pertany als Galliformes, però les característiques altament derivades del crani justifiquen el seu emplaçament a una nova família, extingida, Sylviornithidae. El crani de Syluiornis està extremadament eixamplat i dorsoventralment aplanat, mentre que el rostrum és massís, lateralment comprimit, dorsoventralment aixecat i mostra unes cristae tomiales molt fondes. El rostrum exhibeix un ornament ossi gran. La mandíbula mostra una símfisi molt allargada, les branques laterals també presenten unes cristae tomiales fondes, i la part posterior de la mandíbula és molt gruixada. Es discuteix el possible origen i l'alimentació de Syluiornis. Paraules clau: Aves, Galliformes, Extinció, Holocè, Nova Caledònia. Abstract The skull of Syluiornis shows a completely mobile craniorostral articulation, with two articular condyles situated on the rostrum, which insert into two elongated articular surfaces on the cranium. -

South Africa: Magoebaskloof and Kruger National Park Custom Tour Trip Report
SOUTH AFRICA: MAGOEBASKLOOF AND KRUGER NATIONAL PARK CUSTOM TOUR TRIP REPORT 24 February – 2 March 2019 By Jason Boyce This Verreaux’s Eagle-Owl showed nicely one late afternoon, puffing up his throat and neck when calling www.birdingecotours.com [email protected] 2 | TRIP REPORT South Africa: Magoebaskloof and Kruger National Park February 2019 Overview It’s common knowledge that South Africa has very much to offer as a birding destination, and the memory of this trip echoes those sentiments. With an itinerary set in one of South Africa’s premier birding provinces, the Limpopo Province, we were getting ready for a birding extravaganza. The forests of Magoebaskloof would be our first stop, spending a day and a half in the area and targeting forest special after forest special as well as tricky range-restricted species such as Short-clawed Lark and Gurney’s Sugarbird. Afterwards we would descend the eastern escarpment and head into Kruger National Park, where we would make our way to the northern sections. These included Punda Maria, Pafuri, and the Makuleke Concession – a mouthwatering birding itinerary that was sure to deliver. A pair of Woodland Kingfishers in the fever tree forest along the Limpopo River Detailed Report Day 1, 24th February 2019 – Transfer to Magoebaskloof We set out from Johannesburg after breakfast on a clear Sunday morning. The drive to Polokwane took us just over three hours. A number of birds along the way started our trip list; these included Hadada Ibis, Yellow-billed Kite, Southern Black Flycatcher, Village Weaver, and a few brilliant European Bee-eaters. -
Dwarf Thistle, Cirsi
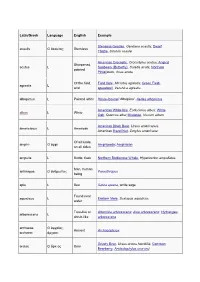
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Attagis Gayi Geoffroy Saint Hilaire Y Lesson, 1831
FICHA DE ANTECEDENTES DE ESPECIE Id especie: NOMBRE CIENTÍFICO: Attagis gayi Geoffroy Saint Hilaire y Lesson, 1831 NOMBRE COMÚN: Perdicita cordillerana, tortolón, agachona grande, Rufous-bellied Seedsnipe Fotografía de Attagis gayi (AUTOR, Roberto Villablanca) Reino: Animalia Orden: Charadriiformes Phyllum/División: Chordata Familia: Thinocoridae Clase: Aves Género: Attagis Sinonimia: Nota Taxonómica: Se reconocen tres subespecies (Fjeldsa 1996): • Attagis gayi latreillii , Lesson 1831. Habita el norte de Ecuador • Attagis gayi simonsi , Chubb 1918, centro de Perú, hasta el norte de Chile, oeste de Bolivia y noroeste de Argentina. • Attagis gayi gayi , Geoffroy Saint Hilaire y Lesson 1831, Andes de Chile y Argentina, desde Antofagasta y Salta hasta Tierra del Fuego. ANTECEDENTES GENERALES Aspectos Morfológicos Longitud 27 a 31 cm, es la más grande de los representantes de la familia, no presenta dimorfismo sexual y tiene un cuerpo grande, con cabeza pequeña y piernas cortas, Su plumaje es muy críptico, con una coloración general parda, con ocre y rojizo en el abdomen (Martínez & González 2004). Color general por encima de un gris ceniza con rayitas o flechas concéntricas de un negro oscuro. Cubiertas alares más rufas y también moteadas de flechas negras. Primarias pardo oscuro, negruzcas en su barba externa. Supracaudales y rectrices rufo pálido con vermiculaciones negras. Garganta color isabelino, lados de ésta, cuello y parte superior del pecho de fondo rufo pálido con vermiculaciones negras concéntricas. Toda la parte inferior restante de un canela pálido u oscuro en algunos ejemplares. Subcaudales de igual coloración y en algunos ejemplares con verniculaciones transversales negras. Axilares y subalares canela pálido (Jaramillo 2005). Aspectos Reproductivos Nidifican en el suelo, los nidos son rudimentarios. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

TNP SOK 2011 Internet
GARDEN ROUTE NATIONAL PARK : THE TSITSIKAMMA SANP ARKS SECTION STATE OF KNOWLEDGE Contributors: N. Hanekom 1, R.M. Randall 1, D. Bower, A. Riley 2 and N. Kruger 1 1 SANParks Scientific Services, Garden Route (Rondevlei Office), PO Box 176, Sedgefield, 6573 2 Knysna National Lakes Area, P.O. Box 314, Knysna, 6570 Most recent update: 10 May 2012 Disclaimer This report has been produced by SANParks to summarise information available on a specific conservation area. Production of the report, in either hard copy or electronic format, does not signify that: the referenced information necessarily reflect the views and policies of SANParks; the referenced information is either correct or accurate; SANParks retains copies of the referenced documents; SANParks will provide second parties with copies of the referenced documents. This standpoint has the premise that (i) reproduction of copywrited material is illegal, (ii) copying of unpublished reports and data produced by an external scientist without the author’s permission is unethical, and (iii) dissemination of unreviewed data or draft documentation is potentially misleading and hence illogical. This report should be cited as: Hanekom N., Randall R.M., Bower, D., Riley, A. & Kruger, N. 2012. Garden Route National Park: The Tsitsikamma Section – State of Knowledge. South African National Parks. TABLE OF CONTENTS 1. INTRODUCTION ...............................................................................................................2 2. ACCOUNT OF AREA........................................................................................................2 -

Harrier References
Introduction This is the final version of the Harrier's list, no further updates will be made. Grateful thanks to Wietze Janse and Tom Shevlin (www.irishbirds.ie) for the cover images and all those who responded with constructive feedback. All images © the photographers. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2019. IOC World Bird List. Available from: https://www.worldbirdnames.org/ [version 9.1 accessed January 2019]). Final Version Version 1.4 (January 2019). Cover Main image: Western Marsh Harrier. Zevenhoven, Groene Jonker, Netherlands. 3rd May 2011. Picture by Wietze Janse. Vignette: Montagu’s Harrier. Great Saltee Island, Co. Wexford, Ireland. 10th May 2008. Picture by Tom Shevlin. Species Page No. African Marsh Harrier [Circus ranivorus] 8 Black Harrier [Circus maurus] 10 Cinereous Harrier [Circus cinereus] 17 Eastern Marsh Harrier [Circus spilonotus] 6 Hen Harrier [Circus cyaneus] 11 Long-winged Harrier [Circus buffoni] 9 Malagasy Harrier [Circus macrosceles] 9 Montagu's Harrier [Circus pygargus] 20 Northern Harrier [Circus hudsonius] 16 Pallid Harrier [Circus macrourus] 18 Papuan Harrier [Circus spilothorax] 7 Pied Harrier [Circus melanoleucos] 20 Réunion Harrier [Circus maillardi] 9 Spotted Harrier [Circus assimilis] 9 Swamp Harrier [Circus approximans] 7 Western Marsh Harrier [Circus aeruginosus] 4 1 Relevant Publications Balmer, D. et al. 2013. Bird Atlas 2001-11: The breeding and wintering birds of Britain and Ireland. BTO Books, Thetford. Beaman, M.