Avian Nesting and Roosting on Glaciers at High Elevation, Cordillera Vilcanota, Peru
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Freshwater Diatoms in the Sajama, Quelccaya, and Coropuna Glaciers of the South American Andes
Diatom Research ISSN: 0269-249X (Print) 2159-8347 (Online) Journal homepage: http://www.tandfonline.com/loi/tdia20 Freshwater diatoms in the Sajama, Quelccaya, and Coropuna glaciers of the South American Andes D. Marie Weide , Sherilyn C. Fritz, Bruce E. Brinson, Lonnie G. Thompson & W. Edward Billups To cite this article: D. Marie Weide , Sherilyn C. Fritz, Bruce E. Brinson, Lonnie G. Thompson & W. Edward Billups (2017): Freshwater diatoms in the Sajama, Quelccaya, and Coropuna glaciers of the South American Andes, Diatom Research, DOI: 10.1080/0269249X.2017.1335240 To link to this article: http://dx.doi.org/10.1080/0269249X.2017.1335240 Published online: 17 Jul 2017. Submit your article to this journal Article views: 6 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tdia20 Download by: [Lund University Libraries] Date: 19 July 2017, At: 08:18 Diatom Research,2017 https://doi.org/10.1080/0269249X.2017.1335240 Freshwater diatoms in the Sajama, Quelccaya, and Coropuna glaciers of the South American Andes 1 1 2 3 D. MARIE WEIDE ∗,SHERILYNC.FRITZ,BRUCEE.BRINSON, LONNIE G. THOMPSON & W. EDWARD BILLUPS2 1Department of Earth and Atmospheric Sciences, University of Nebraska-Lincoln, Lincoln, NE, USA 2Department of Chemistry, Rice University, Houston, TX, USA 3School of Earth Sciences and Byrd Polar and Climate Research Center, The Ohio State University, Columbus, OH, USA Diatoms in ice cores have been used to infer regional and global climatic events. These archives offer high-resolution records of past climate events, often providing annual resolution of environmental variability during the Late Holocene. -

Phylogenetic Reanalysis of Strauch's Osteological Data Set for The
TheCondor97:174-196 0 The Cooper Ornithological Society 1995 PHYLOGENETIC REANALYSIS OF STRAUCH’S OSTEOLOGICAL DATA SET FOR THE CHARADRIIFORMES PHILIP c. CHU Department of Biology and Museum of Zoology The University of Michigan, Ann Arbor, MI 48109 Abstract. Strauch’s (1978) compatibility analysisof relationshipsamong the shorebirds (Charadriifonnes) was the first study to examine the full range of charadriifonn taxa in a reproducibleway. SubsequentlyMickevich and Parenti (1980) leveled seriouscharges against Strauch’s characters,method of phylogenetic inference, and results. To account for these charges,Strauch ’s characterswere re-examined and recoded, and parsimony analyseswere performed on the revised matrix. A parsimony analysison 74 taxa from the revised matrix yielded 855 shortesttrees, each length = 286 and consistencyindex = 0.385. In each shortest tree there were two major lineages,a lineageof sandpiper-likebirds and a lineageof plover- like birds; the two formed a monophyletic group, with the auks (Alcidae) being that group’s sister taxon. The shortest trees were then compared with other estimates of shorebird re- lationships, comparison suggestingthat the chargesagainst Strauch’s results may have re- sulted from the Mickevich and Parenti decisions to exclude much of Strauch’s character evidence. Key words: Charadrilformes; phylogeny; compatibility analysis: parsimony analysis; tax- onomic congruence. INTRODUCTION Strauch scored 227 charadriiform taxa for 70 The investigation of evolutionary relationships characters. Sixty-three of the characters were among shorebirds (Aves: Charadriiformes) has a taken from either the skull or postcranial skel- long history (reviewed in Sibley and Ahlquist eton; the remaining seven involved the respec- 1990). Almost all studies used morphology to tive origins of three neck muscles, as published make inferences about shared ancestry; infer- in Burton (1971, 1972, 1974) and Zusi (1962). -

SPLITS, LUMPS and SHUFFLES Splits, Lumps and Shuffles Alexander C
>> SPLITS, LUMPS AND SHUFFLES Splits, lumps and shuffles Alexander C. Lees This series focuses on recent taxonomic proposals—be they entirely new species, splits, lumps or reorganisations—that are likely to be of greatest interest to birders. This latest instalment includes a new Scytalopus tapaculo and a new subspecies of Three-striped Warbler, reviews of species limits in Grey-necked Wood Rails and Pearly Parakeets and comprehensive molecular studies of Buff-throated Woodcreepers, Sierra Finches, Red-crowned Ant Tanagers and Siskins. Get your lists out! Splits proposed for Grey- Pearly Parakeet is two species necked Wood Rails The three subspecies of Pearly Parakeet Pyrrhura lepida form a species complex with Crimson- The Grey-necked Wood Rail Aramides cajaneus bellied Parakeet P. perlata and replace each other is both the most widespread (occurring from geographically across a broad swathe of southern Mexico to Argentina) and the only polytypic Amazonia east of the Madeira river all the way member of its genus. Although all populations to the Atlantic Ocean. Understanding the nature are ‘diagnosable’ in having an entirely grey neck of this taxonomic variation is an important task, and contrasting chestnut chest, there is much as collectively their range sits astride much of variation in the colours of the nape, lower chest the Amazonian ‘Arc of Deforestation’ and the and mantle, differences amongst which have led to broadly-defined Brazilian endemic Pearly Parakeet the recognition of nine subspecies. Marcondes and is already considered to be globally Vulnerable. Silveira (2015) recently explored the taxonomy of Somenzari and Silveira (2015) recently investigated Grey-necked Wood Rails based on morphological the taxonomy of the three lepida subspecies (the and vocal characteristics using a sample of 800 nominate P. -
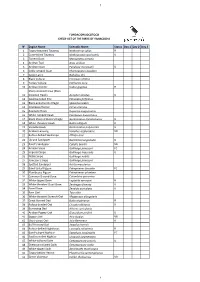
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

Appendix 1: Maps and Plans Appendix184 Map 1: Conservation Categories for the Nominated Property
Appendix 1: Maps and Plans Appendix184 Map 1: Conservation Categories for the Nominated Property. Los Alerces National Park, Argentina 185 Map 2: Andean-North Patagonian Biosphere Reserve: Context for the Nominated Proprty. Los Alerces National Park, Argentina 186 Map 3: Vegetation of the Valdivian Ecoregion 187 Map 4: Vegetation Communities in Los Alerces National Park 188 Map 5: Strict Nature and Wildlife Reserve 189 Map 6: Usage Zoning, Los Alerces National Park 190 Map 7: Human Settlements and Infrastructure 191 Appendix 2: Species Lists Ap9n192 Appendix 2.1 List of Plant Species Recorded at PNLA 193 Appendix 2.2: List of Animal Species: Mammals 212 Appendix 2.3: List of Animal Species: Birds 214 Appendix 2.4: List of Animal Species: Reptiles 219 Appendix 2.5: List of Animal Species: Amphibians 220 Appendix 2.6: List of Animal Species: Fish 221 Appendix 2.7: List of Animal Species and Threat Status 222 Appendix 3: Law No. 19,292 Append228 Appendix 4: PNLA Management Plan Approval and Contents Appendi242 Appendix 5: Participative Process for Writing the Nomination Form Appendi252 Synthesis 252 Management Plan UpdateWorkshop 253 Annex A: Interview Guide 256 Annex B: Meetings and Interviews Held 257 Annex C: Self-Administered Survey 261 Annex D: ExternalWorkshop Participants 262 Annex E: Promotional Leaflet 264 Annex F: Interview Results Summary 267 Annex G: Survey Results Summary 272 Annex H: Esquel Declaration of Interest 274 Annex I: Trevelin Declaration of Interest 276 Annex J: Chubut Tourism Secretariat Declaration of Interest 278 -

Attagis Gayi Geoffroy Saint Hilaire Y Lesson, 1831
FICHA DE ANTECEDENTES DE ESPECIE Id especie: NOMBRE CIENTÍFICO: Attagis gayi Geoffroy Saint Hilaire y Lesson, 1831 NOMBRE COMÚN: Perdicita cordillerana, tortolón, agachona grande, Rufous-bellied Seedsnipe Fotografía de Attagis gayi (AUTOR, Roberto Villablanca) Reino: Animalia Orden: Charadriiformes Phyllum/División: Chordata Familia: Thinocoridae Clase: Aves Género: Attagis Sinonimia: Nota Taxonómica: Se reconocen tres subespecies (Fjeldsa 1996): • Attagis gayi latreillii , Lesson 1831. Habita el norte de Ecuador • Attagis gayi simonsi , Chubb 1918, centro de Perú, hasta el norte de Chile, oeste de Bolivia y noroeste de Argentina. • Attagis gayi gayi , Geoffroy Saint Hilaire y Lesson 1831, Andes de Chile y Argentina, desde Antofagasta y Salta hasta Tierra del Fuego. ANTECEDENTES GENERALES Aspectos Morfológicos Longitud 27 a 31 cm, es la más grande de los representantes de la familia, no presenta dimorfismo sexual y tiene un cuerpo grande, con cabeza pequeña y piernas cortas, Su plumaje es muy críptico, con una coloración general parda, con ocre y rojizo en el abdomen (Martínez & González 2004). Color general por encima de un gris ceniza con rayitas o flechas concéntricas de un negro oscuro. Cubiertas alares más rufas y también moteadas de flechas negras. Primarias pardo oscuro, negruzcas en su barba externa. Supracaudales y rectrices rufo pálido con vermiculaciones negras. Garganta color isabelino, lados de ésta, cuello y parte superior del pecho de fondo rufo pálido con vermiculaciones negras concéntricas. Toda la parte inferior restante de un canela pálido u oscuro en algunos ejemplares. Subcaudales de igual coloración y en algunos ejemplares con verniculaciones transversales negras. Axilares y subalares canela pálido (Jaramillo 2005). Aspectos Reproductivos Nidifican en el suelo, los nidos son rudimentarios. -

Te Oribatid Mites
Te Oribatid Mites (Acari: Oribatida) C O P A of high-Andean Cushion Peatlands Cologne Paleoecology Jonathan Hense1,4, Karsten Schittek1,2, Markus Forbriger3, & Michael Bonkowski4 University of Cologne 1Cologne Paleoecology Working Group (COPA) 2Seminar for Geographical Education 3Geographical Institute - Quaternary Sciences & Geomorphology 4Zoological Institute - Terrestrial Ecology 80°W 70°W Introduction Results Cushion peatlands (locally referred to as bofedales), occur- In total, 17 Oribatid mite taxa could be identifed for CLP. ring besides streams, lakes and springs in the Puna ecoregion, 4 species (Neoamerioppia notata, Ceratozetes nigrisetosus, are a unique ecosystem adopted to the harsh environmental Jugatala armata, Zetomimus furcatus) could be proven for 10°S 10°S conditions of the high Andes >3.000m a.s.l.. Te inhabit- Peru for the frst time. For all investigated cushion peat- ing Oritabid mite community and the Andean occurence LIMA lands, 37 species from 30 genera and 16 families are re- in general is poorly studied. ported (see Table 1). Of these, 31 species occur only in one ? ? locality. Only 6 species, Camisia khencensis, Jugatala armata Legend LA PAZ (Syn. Edwardzetes armatus), Malaconothrus monodactylus, Cerro Llamoca peatland sampling site is a M. translamellatus, Nanhermannia elegantissima and Tecto- cushion peatland cepheus sp. 20°S Oribatid mite sampling sites 20°S , have been found in two or more localities. No Hammer, 1958 & 1961 Beck, 1963 species has been found at all sites. Covarrubias & Mellado, 2003 Covarrubias, -

Dacninae Species Tree, Part I
Dacninae I: Nemosiini, Conirostrini, & Diglossini Hooded Tanager, Nemosia pileata Cherry-throated Tanager, Nemosia rourei Nemosiini Blue-backed Tanager, Cyanicterus cyanicterus White-capped Tanager, Sericossypha albocristata Scarlet-throated Tanager, Sericossypha loricata Bicolored Conebill, Conirostrum bicolor Pearly-breasted Conebill, Conirostrum margaritae Chestnut-vented Conebill, Conirostrum speciosum Conirostrini White-eared Conebill, Conirostrum leucogenys Capped Conebill, Conirostrum albifrons Giant Conebill, Conirostrum binghami Blue-backed Conebill, Conirostrum sitticolor White-browed Conebill, Conirostrum ferrugineiventre Tamarugo Conebill, Conirostrum tamarugense Rufous-browed Conebill, Conirostrum rufum Cinereous Conebill, Conirostrum cinereum Stripe-tailed Yellow-Finch, Pseudochloris citrina Gray-hooded Sierra Finch, Phrygilus gayi Patagonian Sierra Finch, Phrygilus patagonicus Peruvian Sierra Finch, Phrygilus punensis Black-hooded Sierra Finch, Phrygilus atriceps Gough Finch, Rowettia goughensis White-bridled Finch, Melanodera melanodera Yellow-bridled Finch, Melanodera xanthogramma Inaccessible Island Finch, Nesospiza acunhae Nightingale Island Finch, Nesospiza questi Wilkins’s Finch, Nesospiza wilkinsi Saffron Finch, Sicalis flaveola Grassland Yellow-Finch, Sicalis luteola Orange-fronted Yellow-Finch, Sicalis columbiana Sulphur-throated Finch, Sicalis taczanowskii Bright-rumped Yellow-Finch, Sicalis uropigyalis Citron-headed Yellow-Finch, Sicalis luteocephala Patagonian Yellow-Finch, Sicalis lebruni Greenish Yellow-Finch, -

Holocene Glacier Fluctuations
Quaternary Science Reviews 111 (2015) 9e34 Contents lists available at ScienceDirect Quaternary Science Reviews journal homepage: www.elsevier.com/locate/quascirev Invited review Holocene glacier fluctuations * Olga N. Solomina a, b, , Raymond S. Bradley c, Dominic A. Hodgson d, Susan Ivy-Ochs e, f, Vincent Jomelli g, Andrew N. Mackintosh h, Atle Nesje i, j, Lewis A. Owen k, Heinz Wanner l, Gregory C. Wiles m, Nicolas E. Young n a Institute of Geography RAS, Staromonetny-29, 119017, Staromonetny, Moscow, Russia b Tomsk State University, Tomsk, Russia c Department of Geosciences, University of Massachusetts, Amherst, MA 012003, USA d British Antarctic Survey, High Cross, Madingley Road, Cambridge CB3 0ET, UK e Institute of Particle Physics, ETH Zurich, 8093 Zurich, Switzerland f Institute of Geography, University of Zurich, 8057 Zurich, Switzerland g Universite Paris 1 Pantheon-Sorbonne, CNRS Laboratoire de Geographie Physique, 92195 Meudon, France h Antarctic Research Centre, Victoria University Wellington, New Zealand i Department of Earth Science, University of Bergen, N-5020 Bergen, Norway j Uni Research Klima, Bjerknes Centre for Climate Research, N-5020 Bergen Norway k Department of Geology, University of Cincinnati, Cincinnati, OH 45225, USA l Institute of Geography and Oeschger Centre for Climate Change Research, University of Bern, Switzerland m Department of Geology, The College of Wooster, Wooster, OH 44691, USA n Lamont-Doherty Earth Observatory, Columbia University, Palisades, NY, USA article info abstract Article history: A global overview of glacier advances and retreats (grouped by regions and by millennia) for the Received 15 July 2014 Holocene is compiled from previous studies. The reconstructions of glacier fluctuations are based on Received in revised form 1) mapping and dating moraines defined by 14C, TCN, OSL, lichenometry and tree rings (discontinuous 22 November 2014 records/time series), and 2) sediments from proglacial lakes and speleothems (continuous records/ Accepted 27 November 2014 time series). -

ECUADOR: the Andes Introtour and High Andes Extension 10Th- 19Th November 2019
Tropical Birding - Trip Report Ecuador: The Andes Introtour, November 2019 A Tropical Birding SET DEPARTURE tour ECUADOR: The Andes Introtour and High Andes Extension th th 10 - 19 November 2019 TOUR LEADER: Jose Illanes Report and photos by Jose Illanes Andean Condor from Antisana National Park This is one Tropical Birding’s most popular tours and I have guided it numerous times. It’s always fun and offers so many memorable birds. Ecuador is a wonderful country to visit with beautiful landscapes, rich culture, and many friendly people that you will meet along the way. Some of the highlights picked by the group were Andean Condor, White-throated Screech-Owl, Giant Antpitta, Jameson’s Snipe, Giant Hummingbird, Black-tipped Cotinga, Sword-billed Hummingbird, Club-winged Manakin, Lyre-tailed Nightjar, Lanceolated Monklet, Flame-faced Tanager, Toucan Barbet, Violet-tailed Sylph, Undulated Antpitta, Andean Gull, Blue-black Grassquit, and the attractive Blue-winged Mountain-Tanager. Our total species count on the trip (including the extension) was around 368 seen and 31 heard only. www.tropicalbirding.com +1-409-515-9110 [email protected] p.1 Tropical Birding - Trip Report Ecuador: The Andes Introtour, November 2019 Torrent Duck at Guango Lodge on the extension November 11: After having arrived in Quito the night before, we had our first birding this morning in the Yanacocha Reserve owned by the Jocotoco Foundation, which is not that far from Ecuador’s capital. Our first stop was along the entrance road near a water pumping station, where we started out by seeing Streak- throated Bush-Tyrant, Brown-backed Chat-Tyrant, Cinereous Conebill, White-throated Tyrannulet, a very responsive Superciliaried Hemispingus, Black-crested Warbler, and the striking Crimson-mantled Woodpecker. -

An Update of Wallacels Zoogeographic Regions of the World
REPORTS To examine the temporal profile of ChC produc- specification of a distinct, and probably the last, 3. G. A. Ascoli et al., Nat. Rev. Neurosci. 9, 557 (2008). tion and their correlation to laminar deployment, cohort in this lineage—the ChCs. 4. J. Szentágothai, M. A. Arbib, Neurosci. Res. Program Bull. 12, 305 (1974). we injected a single pulse of BrdU into pregnant A recent study demonstrated that progeni- CreER 5. P. Somogyi, Brain Res. 136, 345 (1977). Nkx2.1 ;Ai9 females at successive days be- tors below the ventral wall of the lateral ventricle 6. L. Sussel, O. Marin, S. Kimura, J. L. Rubenstein, tween E15 and P1 to label mitotic progenitors, (i.e., VGZ) of human infants give rise to a medial Development 126, 3359 (1999). each paired with a pulse of tamoxifen at E17 to migratory stream destined to the ventral mPFC 7. S. J. Butt et al., Neuron 59, 722 (2008). + 18 8. H. Taniguchi et al., Neuron 71, 995 (2011). label NKX2.1 cells (Fig. 3A). We first quanti- ( ). Despite species differences in the develop- 9. L. Madisen et al., Nat. Neurosci. 13, 133 (2010). fied the fraction of L2 ChCs (identified by mor- mental timing of corticogenesis, this study and 10. J. Szabadics et al., Science 311, 233 (2006). + phology) in mPFC that were also BrdU+. Although our findings raise the possibility that the NKX2.1 11. A. Woodruff, Q. Xu, S. A. Anderson, R. Yuste, Front. there was ChC production by E15, consistent progenitors in VGZ and their extended neurogenesis Neural Circuits 3, 15 (2009). -

Satellite Imagery Reveals New Critical Habitat for Endangered Bird Species in the High Andes of Peru
Vol. 13: 145–157, 2011 ENDANGERED SPECIES RESEARCH Published online February 16 doi: 10.3354/esr00323 Endang Species Res OPENPEN ACCESSCCESS Satellite imagery reveals new critical habitat for Endangered bird species in the high Andes of Peru Phred M. Benham1, Elizabeth J. Beckman1, Shane G. DuBay1, L. Mónica Flores2, Andrew B. Johnson1, Michael J. Lelevier1, C. Jonathan Schmitt1, Natalie A. Wright1, Christopher C. Witt1,* 1Museum of Southwestern Biology and Department of Biology, University of New Mexico, Albuquerque, New Mexico 87110, USA 2Centro de Ornitologia y Biodiversidad (CORBIDI), Urb. Huertos de San Antonio, Surco, Lima, Peru ABSTRACT: High-resolution satellite imagery that is freely available on Google Earth provides a powerful tool for quantifying habitats that are small in extent and difficult to detect by medium- resolution Landsat imagery. Relictual Polylepis forests of the high Andes are of critical importance to several globally threatened bird species, but, despite growing international attention to Polylepis conservation, many gaps remain in our knowledge of its distribution. We examined high-resolution satellite imagery in Google Earth to search for new areas of Polylepis in south-central Peru that potentially support Polylepis-specialist bird species. In central Apurímac an extensive region of high- resolution satellite imagery contained 127 Polylepis fragments, totaling 683.15 ha of forest ranging from 4000 to 4750 m a.s.l. Subsequent fieldwork confirmed the presence of mature Polylepis forest and all 6 Polylepis-specialist bird species, 5 of which are considered globally threatened. Our find- ings (1) demonstrate the utility of Google Earth for applied conservation and (2) suggest improved prospects for the persistence of the Polylepis-associated avifauna.