Ecology of Wattled Ibis (Bostrychia Carunculata) in Relation to Land Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comments on the Ornithology of Nigeria, Including Amendments to the National List
Robert J. Dowsett 154 Bull. B.O.C. 2015 135(2) Comments on the ornithology of Nigeria, including amendments to the national list by Robert J. Dowsett Received 16 December 2014 Summary.—This paper reviews the distribution of birds in Nigeria that were not treated in detail in the most recent national avifauna (Elgood et al. 1994). It clarifies certain range limits, and recommends the addition to the Nigerian list of four species (African Piculet Verreauxia africana, White-tailed Lark Mirafra albicauda, Western Black-headed Batis Batis erlangeri and Velvet-mantled Drongo Dicrurus modestus) and the deletion (in the absence of satisfactory documentation) of six others (Olive Ibis Bostrychia olivacea, Lesser Short-toed Lark Calandrella rufescens, Richard’s Pipit Anthus richardi, Little Grey Flycatcher Muscicapa epulata, Ussher’s Flycatcher M. ussheri and Rufous-winged Illadopsis Illadopsis rufescens). Recent research in West Africa has demonstrated the need to clarify the distributions of several bird species in Nigeria. I have re-examined much of the literature relating to the country, analysed the (largely unpublished) collection made by Boyd Alexander there in 1904–05 (in the Natural History Museum, Tring; NHMUK), and have reviewed the data available in the light of our own field work in Ghana (Dowsett-Lemaire & Dowsett 2014), Togo (Dowsett-Lemaire & Dowsett 2011a) and neighbouring Benin (Dowsett & Dowsett- Lemaire 2011, Dowsett-Lemaire & Dowsett 2009, 2010, 2011b). The northern or southern localities of species with limited ranges in Nigeria were not always detailed by Elgood et al. (1994), although such information is essential for understanding distribution patterns and future changes. For many Guineo-Congolian forest species their northern limit in West Africa lies on the escarpment of the Jos Plateau, especially Nindam Forest Reserve, Kagoro. -

South Africa: Magoebaskloof and Kruger National Park Custom Tour Trip Report
SOUTH AFRICA: MAGOEBASKLOOF AND KRUGER NATIONAL PARK CUSTOM TOUR TRIP REPORT 24 February – 2 March 2019 By Jason Boyce This Verreaux’s Eagle-Owl showed nicely one late afternoon, puffing up his throat and neck when calling www.birdingecotours.com [email protected] 2 | TRIP REPORT South Africa: Magoebaskloof and Kruger National Park February 2019 Overview It’s common knowledge that South Africa has very much to offer as a birding destination, and the memory of this trip echoes those sentiments. With an itinerary set in one of South Africa’s premier birding provinces, the Limpopo Province, we were getting ready for a birding extravaganza. The forests of Magoebaskloof would be our first stop, spending a day and a half in the area and targeting forest special after forest special as well as tricky range-restricted species such as Short-clawed Lark and Gurney’s Sugarbird. Afterwards we would descend the eastern escarpment and head into Kruger National Park, where we would make our way to the northern sections. These included Punda Maria, Pafuri, and the Makuleke Concession – a mouthwatering birding itinerary that was sure to deliver. A pair of Woodland Kingfishers in the fever tree forest along the Limpopo River Detailed Report Day 1, 24th February 2019 – Transfer to Magoebaskloof We set out from Johannesburg after breakfast on a clear Sunday morning. The drive to Polokwane took us just over three hours. A number of birds along the way started our trip list; these included Hadada Ibis, Yellow-billed Kite, Southern Black Flycatcher, Village Weaver, and a few brilliant European Bee-eaters. -
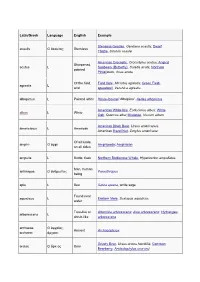
Dwarf Thistle, Cirsi
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Theristicus Caudatus;Argentina
The Condor96:99&1002 Q The cooper omith&gical society1994 BREEDING PERFORMANCE IN RELATION TO NEST-SITE SUBSTRATUM IN A BUFF-NECKED IBIS (THHWTICUS CA UDATUS) POPULATION IN PATAGONIA ’ Jose A. DONAZAR Estacidn Biologica de Doiiana, CSIC, Avda I%@Luisa s.n., 41013 Sevilla, Spain OLGA CEBALL~S Grupo de EstudiosBiologicos Ugarra, Carlos III 19, 31002 Pamplona, Spain ALEJANDRO TRAVAINI AND ALFJANDRO RODRIGUEZ EstacirinBiologica de Doiiana, CSIC Avda M Luisa s.n., 41013 Sevilla, Spain MARTIN FUNES Centro de Ecologia Aplicada de1Neuquen, Casilla de Correos92, 8371 Junin de 10sAndes, Neuquen, Argentina FERNANDO HIRAL~ Estacion Biologicade Dofiana, CSIC Avda M Luisa sn., 41013 Sevilla, Spain Abstract. In northern Argentinean Patagonia, Buff-necked Ibis (Theristicuscaudatus) nest on different substrata:cliffs, trees, and marsh vegetation. According to the ideal-free distribution hypothesis,this polymorphism may be due to the occupationofthe bestbreeding habitats by dominant individuals and the relegation of the subdominant birds to marginal substratawith a lower probability of achieving successfulbreeding. We investigatedwhether there were any variations in the breeding performance among colonies and whether these variations were related to the breeding substratum.Laying date varied from the third week of September to the last week of October, laying occurring earlier in colonies at lower elevations. Clutch size per colony varied between 1.8 and 2.0 (X 1.9, n = 106), but significant differences were not detected among colonies. Brood size per colony varied significantly, rangingbetween 1.3 and 2.0 (52= 1.8, n = 164). The substratumof breedingdid not influence variations in any of these three parameters. The physical condition of the chicks did not vary among substrata,but there was inter-colony variation in broods of two chicks. -

OZ Birds-Hard-Key
OAKLAND ZOO BIRD CROSSWORD HARD Down 1. A long soft feather or arrangement of feathers used by a bird for display. 3. Baby parrots hatch helpless and require parental care. 7. Egyptian Goose genus. Across 8. Fischer’s Lovebirds are ______ nesters. 2. Oakland Zoo conservation partner that rescues, cares for, and 10. Pesticide used from the 1940’s to 1960’s that caused re-homes pet parrots. eggshell thinning in Bald eagles and California Condors. 4. Color of the Cattle Egret’s egg. 11. Oakland Zoo conservation partner that focuses on 5. Food of the California Condor. saving the California Condor population from 6. Group of birds that the Bald Eagles belong to. extinction. 8. The ridged part on the upper mandible of the Malayan Wreathed Hornbill. 12. Nests of the Lesser Flamingo are tall to prevent ______. 9. A group of birds intermediate between geese and ducks. 13. The Blue-bellied Roller is this type of specific 14. Where the Hornbill Nest Project is based. carnivore. 18. Another word to describe a social bird 15. Swahili name for the African Spoonbill. 19. The main predator of the emu. 16. Throat pouch of the Malayan Wreathed Hornbill. 20. Oakland Zoo conservation partner that helps many wild animals, including 17. The Hadada Ibis can be found around wetland ______. parrots. 21. Parrots ingest this to help them eliminate toxins 22. The Lesser Flamingo eats by ______ ______. obtained by eating unripe fruit. 23. Type of symbiotic relationship the Cattle Egret has with large mammals. 24. The Guira Cuckoo will ______, or preen other 25. -

Birds and Mammals of Rwanda's National Parks
Rwanda Birds and mammals of Rwanda’s National Parks Rwanda is quite small, covering an area of around one fifth the size of England. Despite its small size the country is blessed with extensive areas of forest, lakes and swamps which in turn attract a wide species of birds and mammals. Rwanda is a wonderful destination for wildlife tourism and an excellent location to watch Mountain Gorillas. Our tour visits Akagera National Park, which has a mix of wetlands and forest, and the bird-rich Nyungwe Forest National Park. We expect to see almost 25 of the range-restricted Albertine Rift endemics. Birding within Rwanda is still in its infancy and this tour could well bring a few surprise species within the extensive forest systems. Days 1-2: We have a flight to Kigali the capital of Rwanda with arrival on Day 2. Dates Transfer to Akagera National Park in east- Saturday January 15th – Thursday ern Rwanda which is close to the border January 27th 2022 with Tanzania. En route we should Leader: Harriet Kemishiga and local encounter the commoner birds of the coun- guides tryside, including Hamerkop, African Group Size: 8 Sacred and Hadada Ibis, Augur Buzzard, Birds: 300-350 Long-crested Eagle, and Village, Black- headed and Vieillot’s Black Weavers. The journey passes through large tracts of agri- chance of locating Black-chested, Brown cultural areas where Grey-backed Fiscal and Western Banded Snake Eagles, White- resides, whilst patches of marsh and reeds headed Vulture, Ross’s Turaco, Black-col- attract Fan-tailed Widowbird and lared and Red-faced Barbets, Bennett’s Carruther’s Cisticola. -

South Africa Mega Birding III 5Th to 27Th October 2019 (23 Days) Trip Report
South Africa Mega Birding III 5th to 27th October 2019 (23 days) Trip Report The near-endemic Gorgeous Bushshrike by Daniel Keith Danckwerts Tour leader: Daniel Keith Danckwerts Trip Report – RBT South Africa – Mega Birding III 2019 2 Tour Summary South Africa supports the highest number of endemic species of any African country and is therefore of obvious appeal to birders. This South Africa mega tour covered virtually the entire country in little over a month – amounting to an estimated 10 000km – and targeted every single endemic and near-endemic species! We were successful in finding virtually all of the targets and some of our highlights included a pair of mythical Hottentot Buttonquails, the critically endangered Rudd’s Lark, both Cape, and Drakensburg Rockjumpers, Orange-breasted Sunbird, Pink-throated Twinspot, Southern Tchagra, the scarce Knysna Woodpecker, both Northern and Southern Black Korhaans, and Bush Blackcap. We additionally enjoyed better-than-ever sightings of the tricky Barratt’s Warbler, aptly named Gorgeous Bushshrike, Crested Guineafowl, and Eastern Nicator to just name a few. Any trip to South Africa would be incomplete without mammals and our tally of 60 species included such difficult animals as the Aardvark, Aardwolf, Southern African Hedgehog, Bat-eared Fox, Smith’s Red Rock Hare and both Sable and Roan Antelopes. This really was a trip like no other! ____________________________________________________________________________________ Tour in Detail Our first full day of the tour began with a short walk through the gardens of our quaint guesthouse in Johannesburg. Here we enjoyed sightings of the delightful Red-headed Finch, small numbers of Southern Red Bishops including several males that were busy moulting into their summer breeding plumage, the near-endemic Karoo Thrush, Cape White-eye, Grey-headed Gull, Hadada Ibis, Southern Masked Weaver, Speckled Mousebird, African Palm Swift and the Laughing, Ring-necked and Red-eyed Doves. -

Cambodian Journal of Natural History
Cambodian Journal of Natural History Giant ibis census Patterns of salt lick use Protected area revisions Economic contribution of NTFPs New plants, bees and range extensions June 2016 Vol. 2016 No. 1 Cambodian Journal of Natural History ISSN 2226–969X Editors Email: [email protected] • Dr Neil M. Furey, Chief Editor, Fauna & Flora International, Cambodia. • Dr Jenny C. Daltry, Senior Conservation Biologist, Fauna & Flora International, UK. • Dr Nicholas J. Souter, Mekong Case Study Manager, Conservation International, Cambodia. • Dr Ith Saveng, Project Manager, University Capacity Building Project, Fauna & Flora International, Cambodia. International Editorial Board • Dr Stephen J. Browne, Fauna & Flora International, • Dr Sovanmoly Hul, Muséum National d’Histoire Singapore. Naturelle, Paris, France. • Dr Martin Fisher, Editor of Oryx – The International • Dr Andy L. Maxwell, World Wide Fund for Nature, Journal of Conservation, Cambridge, U.K. Cambodia. • Dr L. Lee Grismer, La Sierra University, California, • Dr Brad Pett itt , Murdoch University, Australia. USA. • Dr Campbell O. Webb, Harvard University Herbaria, • Dr Knud E. Heller, Nykøbing Falster Zoo, Denmark. USA. Other peer reviewers for this volume • Prof. Leonid Averyanov, Komarov Botanical Institute, • Neang Thy, Minstry of Environment, Cambodia. Russia. • Dr Nguyen Quang Truong, Institute of Ecology and • Prof. John Blake, University of Florida, USA. Biological Resources, Vietnam. • Dr Stephan Gale, Kadoorie Farm & Botanic Garden, • Dr Alain Pauly, Royal Belgian Institute of Natural Hong Kong. Sciences, Belgium. • Fredéric Goes, Cambodia Bird News, France. • Dr Colin Pendry, Royal Botanical Garden, Edinburgh, • Dr Hubert Kurzweil, Singapore Botanical Gardens, UK. Singapore. • Dr Stephan Risch, Leverkusen, Germany. • Simon Mahood, Wildlife Conservation Society, • Dr Nophea Sasaki, University of Hyogo, Japan. -

Ecophysiological Study of the Intertidal Zonation of the Estuarine Rhodophytes Bostrychia Scorpioides
AUTOR: Raquel Sánchez de Pedro Crespo http://orcid.org/0000-0002-2517-2154 EDITA: Publicaciones y Divulgación Científica. Universidad de Málaga Esta obra está bajo una licencia de Creative Commons Reconocimiento-NoComercial- SinObraDerivada 4.0 Internacional: http://creativecommons.org/licenses/by-nc-nd/4.0/legalcode Cualquier parte de esta obra se puede reproducir sin autorización pero con el reconocimiento y atribución de los autores. No se puede hacer uso comercial de la obra y no se puede alterar, transformar o hacer obras derivadas. Esta Tesis Doctoral está depositada en el Repositorio Institucional de la Universidad de Málaga (RIUMA): riuma.uma.es UNIVERSIDAD DE MÁLAGA FACULTAD DE CIENCIAS DEPARTAMENTO DE ECOLOGÍA Y GEOLOGÍA Área de Ecología Ecophysiological study of the intertidal zonation of the estuarine rhodophytes Bostrychia scorpioides (Hudson) Montagne ex Kützing and Catenella caespitosa (Withering) L. M. Irvine Memoria presentada para optar al grado de Doctor en Ciencias Ambientales por Raquel Sánchez de Pedro Crespo Dirigida por por F. Xavier Niell Castanera y Raquel Carmona Fernández This page was intentionally left blank. i Financial support This PhD project has been financially supported by the following institutions and projects: • Grant of the contract 8.06/44.3089 between the University of Málaga and ENCE (2012- 2014). • International Campus of Excelence of the Sea (CEIMAR), with a contract of "Titulado superior de apoyo a la investigación" (2014-2016). • Contract 8.06/44.4430 between the University of Málaga and ENCE, (2016). • Project “Puntos débiles para el conocimiento del ciclo del Carbono en sistemas estuáricos: relación sumidero-emisión”, CTM 2008-04453, Spanish Ministry of Science and Technology (2012-2016). -

Call Function and Vocal Activity in the Southern Bald Ibis at a Breeding Site
Åòòîëîã³ÿ Á3åðêó 12Â4èï. 2100 258 - 26 CALL FUNCTION AND VOCAL ACTIVITY IN THE SOUTHERN BALD IBIS AT A BREEDING SITE Grzegorz Kopij Abstract. Studies were carried out in a breeding colony at Welgelegen, eastern Free State, South Africa in 1994. Seven different calls in the Southern Bald Ibis were distinguished and their function has been determined: clangor, alarm, barking, copulation, snoring, greeting and begging. In the pre-laying phase, breeding and non- breeding pairs were equally vocal. Among breeding birds females were more (60.0 %) vocal than males (40.0 % of 402 calls recorded), while among non-breeding paired birds the reverse was true (males: 57.5 %, females: 42.5 %; n = 134 calls recorded). At the nest, breeding birds were more vocal during the incubation than during the pre-laying and nestling phases, while non-breeding paired birds are more vocal at the beginning of the breeding season than latter. During the day, breeding birds are more vocal in the morning (7001000; 40.5 %), while non- breeding paired birds are more vocal around the noon (10001400; 50.6 %) and in the evening (16001800; 23.4 %). Key words: Southern Bald Ibis, Geronticus calvus, South Africa, vocalization, vocal activity. Address: Department of Zoology & Ecology, Agricultural University of Wroc³aw, ul. Ko¿uchowska 5b, 51- 631 Wroc³aw, Poland. Ôóíêöèè êðèêîâ è âîêàëüíàÿ àêòèâíîñòü ó ëûñîãî èáèñà â ìåñòàõ ãíåçäîâàíèÿ. - Ã. Êîïèé. - Áåðêóò. 13 (2). 2004. - Èññëåäîâàíèÿ ïðîâîäèëèñü íà îäíîé èç ãíåçäîâûõ êîëîíèé â Âåëãåëåãåíå íà âîñ- òîêå Îðàíæåâîãî Ñâîáîäíîãî Ãîñóäàðñòâà â ÞÀÐ â 1994 ã. Áûëî âûäåëåíî 7 ðàçëè÷íûõ êðèêîâ è îïðåäå- ëåíà èõ ôóíêöèÿ: òðåâîãà, êîïóëÿöèÿ, ïðèâåòñòâèå, âûïðàøèâàíèå è äð. -

Plumage and Behavioral Development of Nestling White Ibises
Wilson Bull., 102(2), 1990, pp. 226-238 PLUMAGE AND BEHAVIORAL DEVELOPMENT OF NESTLING WHITE IBISES TONI L. DE SANTO,’ SUSAN G. MCDOWELL,~ AND KEITH L. BILDSTEIN~ Ans-rticr.- We describe the physical characteristicsand behavioral development OI 17 hand-reared and more than 400 parent-reared nestling White Ibises (Eudocimus albus) hatched in 1985 through 1988 at Pumpkinseed Island, a large colony site in coastal South Carolina. Hatchling ibises are covered with a Pale Neutral Gray to Jet Black natal plumage. About 30% of the hatchlingspossess a tuft of white feathers on their crown, and this pattern persiststhroughout the nestling period. Juvenal plumage, which is complete by 60 days, is mainly Vandyke Brown and Blackish Neutral Gray dorsally and creamy white ventrally. The bill, which is straight at hatching, begins to curve downward at about 14 days. Nestling White Ibises exhibit considerableindividual variation in bill markings from approximately 10 days of age through fledging. Increasingly persistent beggingvocalizations begin within hours of hatching. Nestlings walk on partially extended legsat eight days of age, pirate food from other nestlingsand form crechesat 2 1 days of age, and fledge and join all juvenile and mixed-age feeding flocks at 45-55 days of age. We suggestthat the phenotypic variability in plumage, bill coloration, and beggingcalls we describeenables parental ibises to identify more easily their offspringat the colony site. Received27 Feb. 1989, accepted12 Nov. 1989. Although the plumage and behavioral development of several species of wading birds has been studied in considerable detail (e.g., Hammerkops [&opus umbretta], Wilson et al. 1988; storks, Kahl 1962, 1966; Thomas 1984; herons, Gross 1923; Gavin0 and Dickerman 1972; Juarez and Dickerman 1972; Mc Vaugh 1972,1975; Snow 1974; Merritt 198 l), there are few detailed studies of juvenile ibises. -

How Birds Combat Ectoparasites
The Open Ornithology Journal, 2010, 3, 41-71 41 Open Access How Birds Combat Ectoparasites Dale H. Clayton*,1, Jennifer A. H. Koop1, Christopher W. Harbison1,2, Brett R. Moyer1,3 and Sarah E. Bush1,4 1Department of Biology, University of Utah, Salt Lake City, UT 84112, USA; 2Current address: Biology Department, Siena College, Loudonville, NY, 12211, USA; 3Current address: Providence Day School, Charlotte, NC, 28270, USA; 4Natural History Museum and Biodiversity Research Center, University of Kansas, Lawrence, Kansas 66045, USA Abstract: Birds are plagued by an impressive diversity of ectoparasites, ranging from feather-feeding lice, to feather- degrading bacteria. Many of these ectoparasites have severe negative effects on host fitness. It is therefore not surprising that selection on birds has favored a variety of possible adaptations for dealing with ectoparasites. The functional signifi- cance of some of these defenses has been well documented. Others have barely been studied, much less tested rigorously. In this article we review the evidence - or lack thereof - for many of the purported mechanisms birds have for dealing with ectoparasites. We concentrate on features of the plumage and its components, as well as anti-parasite behaviors. In some cases, we present original data from our own recent work. We make recommendations for future studies that could im- prove our understanding of this poorly known aspect of avian biology. Keywords: Grooming, preening, dusting, sunning, molt, oil, anting, fumigation. INTRODUCTION 2) Mites and ticks (Acari): many families [6-9]. As a class, birds (Aves) are the most thoroughly studied 3) Leeches: four families [10]. group of organisms on earth.