Genetic Diversity and Host Specificity of Blood Parasites of Wading
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bird Checklist
GEESE, SWANS & DUCKS __ Double-crested Cormorant __ Peregrine Falcon __ Laughing Gull __ Franklin’s Gull __ Blk.-bellied Whistling-Duck ANHINGAS RAILS, GALLINULES & __ Ring-billed Gull __ Fulvous Whistling-Duck __ Anhinga COOTS __ Herring Gull __ Grtr. White-fronted Goose __ King Rail __ Least Tern __ Snow Goose PELICANS __ Virginia Rail __ Caspian Tern __ Ross’s Goose __ American White Pelican __ Sora __ Black Tern __ Cackling Goose __ Purple Gallinule __ Forster’s Tern __ Canada Goose BITTERNS & HERONS __ Common Gallinule __ Tundra Swan __ American Bittern __ American Coot PIGEONS & DOVES __ Wood Duck __ Least Bittern __ Rock Pigeon __ Gadwall __ Great Blue Heron CRANES __ Eurasian Collared-Dove __ American Wigeon __ Great Egret __ Sandhill Crane __ White-winged Dove __ American Black Duck __ Snowy Egret __ Mourning Dove BIRD CHECKLIST __ Mallard __ Little Blue Heron PLOVERS __ Blue-winged Teal __ Tricolored Heron __ Black-bellied Plover CUCKOOS & ANIS __ Northern Shoveler __ Cattle Egret __ American Golden-Plover __ Yellow-billed Cuckoo __ Northern Pintail __ Green Heron __ Semipalmated Plover __ Black-billed Cuckoo ara Wildlife is a great place to see the __ Green-winged Teal __ Black-crowned Night-Heron __ Killdeer beautiful and varied bird life of the __ Canvasback __ Yell.-crowned Night-Heron BARN OWLS __ Redhead STILTS & AVOCETS __ Barn Owl Mississippi River valley. Its bottomland __ Ring-necked Duck IBISES & SPOONBILLS __ Black-necked Stilt Tforests, wetlands, river shoreline, sloughs and __ Greater Scaup __ White Ibis __ American Avocet TYPICAL OWLS other habitats harbor Wood Storks, Roseate __ Lesser Scaup __ Glossy Ibis __ Eastern Screech-Owl __ Long-tailed Duck __ White-faced Ibis SANDPIPERS __ Great Horned Owl Spoonbills, Bald Eagles, Prothonotary __ Bufflehead __ Roseate Spoonbill __ Spotted Sandpiper __ Barred Owl Warblers, Painted Buntings and many other __ Common Goldeneye __ Solitary Sandpiper __ Short-eared Owl __ Hooded Merganser VULTURES __ Greater Yellowlegs species. -

156 Glossy Ibis
Text and images extracted from Marchant, S. & Higgins, P.J. (co-ordinating editors) 1990. Handbook of Australian, New Zealand & Antarctic Birds. Volume 1, Ratites to ducks; Part B, Australian pelican to ducks. Melbourne, Oxford University Press. Pages 953, 1071-1 078; plate 78. Reproduced with the permission of Bird life Australia and Jeff Davies. 953 Order CICONIIFORMES Medium-sized to huge, long-legged wading birds with well developed hallux or hind toe, and large bill. Variations in shape of bill used for recognition of sub-families. Despite long legs, walk rather than run and escape by flying. Five families of which three (Ardeidae, Ciconiidae, Threskiornithidae) represented in our region; others - Balaenicipitidae (Shoe-billed Stork) and Scopidae (Hammerhead) - monotypic and exclusively Ethiopian. Re lated to Phoenicopteriformes, which sometimes considered as belonging to same order, and, more distantly, to Anseriformes. Behavioural similarities suggest affinities also to Pelecaniformes (van Tets 1965; Meyerriecks 1966), but close relationship not supported by studies of egg-white proteins (Sibley & Ahlquist 1972). Suggested also, mainly on osteological and other anatomical characters, that Ardeidae should be placed in separate order from Ciconiidae and that Cathartidae (New World vultures) should be placed in same order as latter (Ligon 1967). REFERENCES Ligon, J.D. 1967. Occas. Pap. Mus. Zool. Univ. Mich. 651. Sibley, C. G., & J.E. Ahlquist. 1972. Bull. Peabody Mus. nat. Meyerriecks, A.J. 1966. Auk 83: 683-4. Hist. 39. van Tets, G.F. 1965. AOU orn. Monogr. 2. 1071 Family PLATALEIDAE ibises, spoonbills Medium-sized to large wading and terrestial birds. About 30 species in about 15 genera, divided into two sub families: ibises (Threskiornithinae) and spoonbills (Plataleinae); five species in three genera breeding in our region. -

January 2020.Indd
BROWN PELICAN Photo by Rob Swindell at Melbourne, Florida JANUARY 2020 Editors: Jim Jablonski, Marty Ackermann, Tammy Martin, Cathy Priebe Webmistress: Arlene Lengyel January 2020 Program Tuesday, January 7, 2020, 7 p.m. Carlisle Reservation Visitor Center Gulls 101 Chuck Slusarczyk, Jr. "I'm happy to be presenting my program Gulls 101 to the good people of Black River Audubon. Gulls are notoriously difficult to identify, but I hope to at least get you looking at them a little closer. Even though I know a bit about them, I'm far from an expert in the field and there is always more to learn. The challenge is to know the particular field marks that are most important, and familiarization with the many plumage cycles helps a lot too. No one will come out of this presentation an expert, but I hope that I can at least give you an idea what to look for. At the very least, I hope you enjoy the photos. Looking forward to seeing everyone there!” Chuck Slusarczyk is an avid member of the Ohio birding community, and his efforts to assist and educate novice birders via social media are well known, yet he is the first to admit that one never stops learning. He has presented a number of programs to Black River Audubon, always drawing a large, appreciative gathering. 2019 Wellington Area Christmas Bird Count The Wellington-area CBC will take place Saturday, December 28, 2019. Meet at the McDonald’s on Rt. 58 at 8:00 a.m. The leader is Paul Sherwood. -

The Importance of Rice Fields for Glossy Ibis (Plegadis Falcinellus
Biological Conservation 148 (2012) 19–27 Contents lists available at SciVerse ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon The importance of rice fields for glossy ibis (Plegadis falcinellus): Management recommendations derived from an individual-based model ⇑ Gregorio M. Toral a, , Richard A. Stillman b, Simone Santoro a, Jordi Figuerola a a Department of Wetland Ecology, Doñana Biological Station, CSIC, Avda. Américo Vespucio s/n 41092, Seville, Spain b School of Applied Sciences, Bournemouth University, Talbot Campus, Poole, Dorset BH12 5BB, UK article info abstract Article history: Artificial wetlands provide alternative habitats for waterbirds. The Doñana rice fields (SW Spain) are Received 16 May 2011 extensively used as a foraging site by the glossy ibis (Plegadis falcinellus). The aim of this study was to Received in revised form 26 January 2012 develop an individual-based model to predict the possible effects of glossy ibis’ population growth, Accepted 1 February 2012 reductions in the rice cultivated area, and changes on the phenology of the management processes of Available online 22 February 2012 the paddies on the mortality rate of the glossy ibis population. We test the hypothesis that the glossy ibis breeding population of Doñana can obtain its energy requirements during the non-breeding season by Keywords: feeding on rice fields alone. Our results show that the glossy ibis population growth is not currently lim- Wetland ited by rice field availability. However, a reduction of 80% would cause mortality rate increases above the Agriculture Crayfish observed mortality (5.9% per year), with values around 10% per year for populations between 50,000 and Waterbird 100,000 birds. -

Bird Vulnerability Assessments
Assessing the vulnerability of native vertebrate fauna under climate change, to inform wetland and floodplain management of the River Murray in South Australia: Bird Vulnerability Assessments Attachment (2) to the Final Report June 2011 Citation: Gonzalez, D., Scott, A. & Miles, M. (2011) Bird vulnerability assessments- Attachment (2) to ‘Assessing the vulnerability of native vertebrate fauna under climate change to inform wetland and floodplain management of the River Murray in South Australia’. Report prepared for the South Australian Murray-Darling Basin Natural Resources Management Board. For further information please contact: Department of Environment and Natural Resources Phone Information Line (08) 8204 1910, or see SA White Pages for your local Department of Environment and Natural Resources office. Online information available at: http://www.environment.sa.gov.au Permissive Licence © State of South Australia through the Department of Environment and Natural Resources. You may copy, distribute, display, download and otherwise freely deal with this publication for any purpose subject to the conditions that you (1) attribute the Department as the copyright owner of this publication and that (2) you obtain the prior written consent of the Department of Environment and Natural Resources if you wish to modify the work or offer the publication for sale or otherwise use it or any part of it for a commercial purpose. Written requests for permission should be addressed to: Design and Production Manager Department of Environment and Natural Resources GPO Box 1047 Adelaide SA 5001 Disclaimer While reasonable efforts have been made to ensure the contents of this publication are factually correct, the Department of Environment and Natural Resources makes no representations and accepts no responsibility for the accuracy, completeness or fitness for any particular purpose of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of or reliance on the contents of this publication. -

South Africa: Magoebaskloof and Kruger National Park Custom Tour Trip Report
SOUTH AFRICA: MAGOEBASKLOOF AND KRUGER NATIONAL PARK CUSTOM TOUR TRIP REPORT 24 February – 2 March 2019 By Jason Boyce This Verreaux’s Eagle-Owl showed nicely one late afternoon, puffing up his throat and neck when calling www.birdingecotours.com [email protected] 2 | TRIP REPORT South Africa: Magoebaskloof and Kruger National Park February 2019 Overview It’s common knowledge that South Africa has very much to offer as a birding destination, and the memory of this trip echoes those sentiments. With an itinerary set in one of South Africa’s premier birding provinces, the Limpopo Province, we were getting ready for a birding extravaganza. The forests of Magoebaskloof would be our first stop, spending a day and a half in the area and targeting forest special after forest special as well as tricky range-restricted species such as Short-clawed Lark and Gurney’s Sugarbird. Afterwards we would descend the eastern escarpment and head into Kruger National Park, where we would make our way to the northern sections. These included Punda Maria, Pafuri, and the Makuleke Concession – a mouthwatering birding itinerary that was sure to deliver. A pair of Woodland Kingfishers in the fever tree forest along the Limpopo River Detailed Report Day 1, 24th February 2019 – Transfer to Magoebaskloof We set out from Johannesburg after breakfast on a clear Sunday morning. The drive to Polokwane took us just over three hours. A number of birds along the way started our trip list; these included Hadada Ibis, Yellow-billed Kite, Southern Black Flycatcher, Village Weaver, and a few brilliant European Bee-eaters. -
Dwarf Thistle, Cirsi
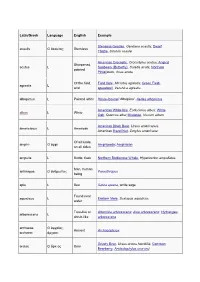
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Behavioural Adaptation of a Bird from Transient Wetland Specialist to an Urban Resident
Behavioural Adaptation of a Bird from Transient Wetland Specialist to an Urban Resident John Martin1,2*, Kris French1, Richard Major2 1 Institute for Conservation Biology and Environmental Management, School of Biological Sciences, University of Wollongong, Wollongong, New South Wales, Australia, 2 Terrestrial Ecology, Australian Museum, Sydney, New South Wales, Australia Abstract Dramatic population increases of the native white ibis in urban areas have resulted in their classification as a nuisance species. In response to community and industry complaints, land managers have attempted to deter the growing population by destroying ibis nests and eggs over the last twenty years. However, our understanding of ibis ecology is poor and a question of particular importance for management is whether ibis show sufficient site fidelity to justify site-level management of nuisance populations. Ibis in non-urban areas have been observed to be highly transient and capable of moving hundreds of kilometres. In urban areas the population has been observed to vary seasonally, but at some sites ibis are always observed and are thought to be behaving as residents. To measure the level of site fidelity, we colour banded 93 adult ibis at an urban park and conducted 3-day surveys each fortnight over one year, then each quarter over four years. From the quarterly data, the first year resighting rate was 89% for females (n = 59) and 76% for males (n = 34); this decreased to 41% of females and 21% of males in the fourth year. Ibis are known to be highly mobile, and 70% of females and 77% of males were observed at additional sites within the surrounding region (up to 50 km distant). -

Roseate Spoonbill Breeding in Camden County: a First State Nesting Record for Georgia
vol. 76 • 3 – 4 THE ORIOLE 65 ROSEATE SPOONBILL BREEDING IN CAMDEN COUNTY: A FIRST STATE NESTING RECORD FOR GEORGIA Timothy Keyes One Conservation Way Brunswick, GA 31520 [email protected] Chris Depkin One Conservation Way Brunswick, GA 31520 [email protected] Jessica Aldridge 6222 Charlie Smith Sr. Hwy St. Marys, GA 31558 [email protected] Introduction We report the northernmost breeding record of Roseate Spoonbill (Platalea ajaja) on the Atlantic Coast of the U.S. Nesting activity has been suspected in Georgia for at least 5 years, but was first confirmed in June 2011 at a large wading bird colony in St. Marys, Camden County, Georgia. Prior to this record, the furthest northern breeding record for Roseate Spoonbill was in St. Augustine, St. John’s County, Florida, approximately 100 km to the south. This record for Georgia continues a trend of northward expansion of Roseate Spoonbill post-breeding dispersal and breeding ranges. Prior to the plume-hunting era of the mid to late 1800s, the eastern population of Roseate Spoonbill was more abundant and widespread than it is today (Dumas 2000), breeding across much of south Florida. Direct persecution and collateral disturbance by egret plume hunters led to a significant range contraction between 1850 and the 1890s (Allen 1942), limiting the eastern population of Roseate Spoonbills to a few sites in Florida Bay by the 1940s. A low of 15 nesting pairs were documented in 1936 (Powell et al. 1989). By the late 1960s, Roseate Spoonbills began to expand out of Florida Bay, slowly reclaiming some of the territory they had lost. -

Namibia & the Okavango
Pel’s Fishing Owl - a pair was found on a wooded island south of Shakawe (Jan-Ake Alvarsson) NAMIBIA & THE OKAVANGO 21 SEPTEMBER – 8 OCTOBER 2017 LEADER: STEVE BRAINE For most of the country the previous three years drought had been broken and although too early for the mi- grants we did however do very well with birding generally. We searched and found all the near endemics as well as the endemic Dune Lark. Besides these we also had a new write-in for the trip! In the floodplains after observing a wonderful Pel’s Fishing Owl we travelled down a side channel of the Okavango River to look for Pygmy Geese, we were lucky and came across several pairs before reaching a dried-out floodplain. Four birds flew out of the reedbeds and looked rather different to the normal weavers of which there were many, a closer look at the two remaining birds revealed a beautiful pair of Cuckoo Finches. These we all enjoyed for a brief period before they followed the other birds which had now disappeared into the reedbeds. Very strong winds on three of the birding days made birding a huge challenge to say the least after not finding the rare and difficult Herero Chat we had to make alternate arrangements at another locality later in the trip. The entire tour from the Hosea Kutako International Airport outside the capital Windhoek and returning there nineteen days later delivered 375 species. Out of these, four birds were seen only by the leader, a further three species were heard but not seen. -

OZ Birds-Hard-Key
OAKLAND ZOO BIRD CROSSWORD HARD Down 1. A long soft feather or arrangement of feathers used by a bird for display. 3. Baby parrots hatch helpless and require parental care. 7. Egyptian Goose genus. Across 8. Fischer’s Lovebirds are ______ nesters. 2. Oakland Zoo conservation partner that rescues, cares for, and 10. Pesticide used from the 1940’s to 1960’s that caused re-homes pet parrots. eggshell thinning in Bald eagles and California Condors. 4. Color of the Cattle Egret’s egg. 11. Oakland Zoo conservation partner that focuses on 5. Food of the California Condor. saving the California Condor population from 6. Group of birds that the Bald Eagles belong to. extinction. 8. The ridged part on the upper mandible of the Malayan Wreathed Hornbill. 12. Nests of the Lesser Flamingo are tall to prevent ______. 9. A group of birds intermediate between geese and ducks. 13. The Blue-bellied Roller is this type of specific 14. Where the Hornbill Nest Project is based. carnivore. 18. Another word to describe a social bird 15. Swahili name for the African Spoonbill. 19. The main predator of the emu. 16. Throat pouch of the Malayan Wreathed Hornbill. 20. Oakland Zoo conservation partner that helps many wild animals, including 17. The Hadada Ibis can be found around wetland ______. parrots. 21. Parrots ingest this to help them eliminate toxins 22. The Lesser Flamingo eats by ______ ______. obtained by eating unripe fruit. 23. Type of symbiotic relationship the Cattle Egret has with large mammals. 24. The Guira Cuckoo will ______, or preen other 25. -

Avifauna from the Teouma Lapita Site, Efate Island, Vanuatu, Including a New Genus and Species of Megapode
Archived at the Flinders Academic Commons: http://dspace.flinders.edu.au/dspace/ ‘This is the peer reviewed version of the following article: Worthy, T., Hawkins, S., Bedford, S. and Spriggs, M. (2015). Avifauna from the Teouma Lapita Site, Efate Island, Vanuatu, including a new genus and species of megapode. Pacific Science, 69(2) pp. 205-254. which has been published in final form at DOI: http://dx.doi.org/10.2984/69.2.6 Article: http://www.bioone.org/doi/full/10.2984/69.2.6 Journal: http://www.uhpress.hawaii.edu/t-pacific-science Copyright 2015, University of Hawaii Press. Published version of the article is reproduced here with permission from the publisher." Avifauna from the Teouma Lapita Site, Efate Island, Vanuatu, Including a New Genus and Species of Megapode1 Trevor H. Worthy,2,5 Stuart Hawkins,3 Stuart Bedford,4 and Matthew Spriggs 4 Abstract: The avifauna of the Teouma archaeological site on Efate in Vanuatu is described. It derives from the Lapita levels (3,000 – 2,800 ybp) and immedi- ately overlying middens extending to ~2,500 ybp. A total of 30 bird species is represented in the 1,714 identified specimens. Twelve species are new records for the island, which, added to previous records, indicates that minimally 39 land birds exclusive of passerines were in the original avifauna. Three-fourths of the 12 newly recorded species appear to have become extinct by the end of Lapita times, 2,800 ybp. The avifauna is dominated by eight species of columbids (47.5% Minimum Number Individuals [MNI ]) including a large extinct tooth- billed pigeon, Didunculus placopedetes from Tonga, and a giant Ducula sp.