The Evolutionary Embryologist Gavin Rylands De Beer (1899–1972)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

February 29, 1964 NATURE PHYSIOLOGY of COLOUR
No. 4922 February 29, 1964 NATURE 859 leads inevitably to cancer. The present state of knowledge fibres" are present. Again (p. 118), von Gelei's evidence of morphology in 'pre-cancerous' lesions does not permit for "m elanin-dispersing fibres" in Phoxinus is inadequat,e an accura te prediction of the direction along which t he and open to ot.her interpretations. There may be a lesion will proceed. This book should undoubtedly be double innervation, but the evidence in favour of it is available in all medical libraries and institutions associ- not n early so good as the present review suggests. ated with cancer research. H. JACKSON Recent work on the p art. played by pituitary hormones in vertebrate colour changes is well discussed. In this connexion, H. R. H ewer should be given the cred it for PHYSIOLOGY OF COLOUR CHANGE first observing the paling of minnows after they have been injected with minnow pituit ary extract. The t'ecent The Control of Chromatophores work of A. K. Kent unfortunately a.ppeared too late to (International Series of Monographs on Pure and Applied be included. Biology: Zoology, No. 14.) By M. Fingerman. Pp. A small blemish in the writing is the use of "feeling". ix+ 184. (Oxford, London, New York and P aris : To say that after experiments an author "felt" that the Pergamon Press, 1963.) 50s. interpretation must be so-and-so is an unhappy ex HE physiology of colour change is a big subject and pression. Hunches (good and bad) are common enough T includes a study of receptor, integrative and effector when we embark on experiments, but " feelings" regarding mechanisms. -

Yasha Gall, Julian Sorell Huxley, 1887-1975
Julian Sorell Huxley, 1887-1975 Yasha Gall Published by Nauka, St. Petersburg, Russia, 2004 Reproduced as an e-book with kind permission of Nauka Science editor: Academician AL Takhtajan Preface by the Science Editor The 20th century was the epoch of discovery in evolutionary biology, marked by many fundamental investigations. Of special significance were the works of AN Severtsov, SS Chetverikov, S Wright, JBS Haldane, G De Beer JS Huxley and R Goldschmidt. Among the general works on evolutionary theory, the one of greatest breadth was Julian Huxley’s book Evolution: The Modern Synthesis (1942). Huxley was one of the first to analyze the mechanisms of macro-evolutionary processes and discuss the evolutionary role of neoteny in terms of developmental genetics (the speed of gene action). Neoteny—the most important mechanism of heritable variation of ontogenesis—has great macro-evolutionary consequences. A Russian translation of Huxley’s book on evolution was prepared for publication by Professor VV Alpatov. The manuscript of the translation had already been sent to production when the August session of the VASKNIL in 1948 burst forth—a destructive moment in the history of biology in our country. The publication was halted, and the manuscript disappeared. I remember well a meeting with Huxley in 1945 in Moscow and Leningrad during the celebratory jubilee at the Academy of Sciences. He was deeply disturbed by the “blossoming” of Lysenkoist obscurantism in biology. It is also important to note that in the 1950s Huxley developed original concepts for controlling the birth rate of the Earth’s population. He openly declared the necessity of forming an international institute at the United Nations, since the global ecosystem already could not sustain the pressure of human “activity” and, together with humanity, might itself die. -

Functional Analysis of the Homeobox Gene Tur-2 During Mouse Embryogenesis
Functional Analysis of The Homeobox Gene Tur-2 During Mouse Embryogenesis Shao Jun Tang A thesis submitted in conformity with the requirements for the Degree of Doctor of Philosophy Graduate Department of Molecular and Medical Genetics University of Toronto March, 1998 Copyright by Shao Jun Tang (1998) National Library Bibriothèque nationale du Canada Acquisitions and Acquisitions et Bibiiographic Services seMces bibliographiques 395 Wellington Street 395, rue Weifington OtbawaON K1AW OttawaON KYAON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence alIowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distri%uteor sell reproduire, prêter' distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fkom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. Functional Analysis of The Homeobox Gene TLr-2 During Mouse Embryogenesis Doctor of Philosophy (1998) Shao Jun Tang Graduate Department of Moiecular and Medicd Genetics University of Toronto Abstract This thesis describes the clonhg of the TLx-2 homeobox gene, the determination of its developmental expression, the characterization of its fiuiction in mouse mesodem and penpheral nervous system (PNS) developrnent, the regulation of nx-2 expression in the early mouse embryo by BMP signalling, and the modulation of the function of nX-2 protein by the 14-3-3 signalling protein during neural development. -
Appendicularia of CTAW
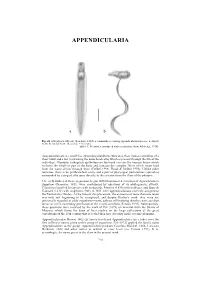
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Review Heterochronic Genes and the Nature of Developmental Time
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology 17, R425–R434, June 5, 2007 ª2007 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2007.03.043 Heterochronic Genes and the Nature of Review Developmental Time Eric G. Moss that have arisen to solve the problem of regulated tim- ing in animal development. Timing is a fundamental issue in development, with Heterochrony and Developmental Timing a range of implications from birth defects to evolu- in Evolution tion. In the roundworm Caenorhabditis elegans, Changes in developmental timing have long been be- the heterochronic genes encode components of lieved to be a major force in the evolution of morphol- a molecular developmental timing mechanism. This ogy [1]. A variety of changes is encompassed by the mechanism functions in diverse cell types through- concept of ‘heterochrony’ — differences in the relative out the animal to specify cell fates at each larval timing of developmental events between two closely stage. MicroRNAs play an important role in this related species. A classic example of heterochrony is mechanism by stage-specifically repressing cell- the axolotl. This salamander reaches sexual maturity fate regulators. Recent studies reveal the surprising without undergoing metamorphosis, such that its complexity surrounding this regulation — for exam- non-gonadal tissues retain larval features of other ple, a positive feedback loop may make the regula- salamanders. Different species of axolotls exhibit ge- tion more robust, and certain components of the netic differences in the production or activity of thyroid mechanism are expressed in brief periods at each hormones that trigger metamorphosis from aquatic stage. -

Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B
Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B. M ü ller, G ü nter P. Wagner, and Werner Callebaut, editors The Evolution of Cognition , edited by Cecilia Heyes and Ludwig Huber, 2000 Origination of Organismal Form: Beyond the Gene in Development and Evolutionary Biology , edited by Gerd B. M ü ller and Stuart A. Newman, 2003 Environment, Development, and Evolution: Toward a Synthesis , edited by Brian K. Hall, Roy D. Pearson, and Gerd B. M ü ller, 2004 Evolution of Communication Systems: A Comparative Approach , edited by D. Kimbrough Oller and Ulrike Griebel, 2004 Modularity: Understanding the Development and Evolution of Natural Complex Systems , edited by Werner Callebaut and Diego Rasskin-Gutman, 2005 Compositional Evolution: The Impact of Sex, Symbiosis, and Modularity on the Gradualist Framework of Evolution , by Richard A. Watson, 2006 Biological Emergences: Evolution by Natural Experiment , by Robert G. B. Reid, 2007 Modeling Biology: Structure, Behaviors, Evolution , edited by Manfred D. Laubichler and Gerd B. M ü ller, 2007 Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability in Human and Animal Communication , edited by Kimbrough D. Oller and Ulrike Griebel, 2008 Functions in Biological and Artifi cial Worlds: Comparative Philosophical Perspectives , edited by Ulrich Krohs and Peter Kroes, 2009 Cognitive Biology: Evolutionary and Developmental Perspectives on Mind, Brain, and Behavior , edited by Luca Tommasi, Mary A. Peterson, and Lynn Nadel, 2009 Innovation in Cultural Systems: Contributions from Evolutionary Anthropology , edited by Michael J. O ’ Brien and Stephen J. Shennan, 2010 The Major Transitions in Evolution Revisited , edited by Brett Calcott and Kim Sterelny, 2011 Transformations of Lamarckism: From Subtle Fluids to Molecular Biology , edited by Snait B. -

Cumulated Bibliography of Biographies of Ocean Scientists Deborah Day, Scripps Institution of Oceanography Archives Revised December 3, 2001
Cumulated Bibliography of Biographies of Ocean Scientists Deborah Day, Scripps Institution of Oceanography Archives Revised December 3, 2001. Preface This bibliography attempts to list all substantial autobiographies, biographies, festschrifts and obituaries of prominent oceanographers, marine biologists, fisheries scientists, and other scientists who worked in the marine environment published in journals and books after 1922, the publication date of Herdman’s Founders of Oceanography. The bibliography does not include newspaper obituaries, government documents, or citations to brief entries in general biographical sources. Items are listed alphabetically by author, and then chronologically by date of publication under a legend that includes the full name of the individual, his/her date of birth in European style(day, month in roman numeral, year), followed by his/her place of birth, then his date of death and place of death. Entries are in author-editor style following the Chicago Manual of Style (Chicago and London: University of Chicago Press, 14th ed., 1993). Citations are annotated to list the language if it is not obvious from the text. Annotations will also indicate if the citation includes a list of the scientist’s papers, if there is a relationship between the author of the citation and the scientist, or if the citation is written for a particular audience. This bibliography of biographies of scientists of the sea is based on Jacqueline Carpine-Lancre’s bibliography of biographies first published annually beginning with issue 4 of the History of Oceanography Newsletter (September 1992). It was supplemented by a bibliography maintained by Eric L. Mills and citations in the biographical files of the Archives of the Scripps Institution of Oceanography, UCSD. -

Eurochordata and Cephalochordata of Persian Gulf & Oman See
Subphylum Urochordata : Tunicates Class Ascidacea Class Larvacea Class Thaliacea Subphylum Cephalochordata : Amphioxus Phylum Chordata : 1 - Subphylum Urochordata 2 Subphylum Cephalochordata (Subphylum Urochordata : Tunicates ) Class Ascidiacea Class Larvacea Class Thaliacea (Subphylum Cephalochordata ) Class Ascidiacea Ascidia Kowalevsky Patricia Kott D. lane David George & Gordon Paterson Herdmania momus savigny Herdmania momus kiamanensis Didemnum candidum savigny Phallusia nigra savigny Styela canopus savigny Class Ascidiacea : Order : Aplousobranchia Family : Polyclinidae : Aplidium cf. rubripunctum C.Monniot & F. Monniot 1997 Polyclinum constellatum Savigny, 1816 Family Didemnidae Didemnum candidum Savigny, 1816 Didemnum cf. granulatum Tokioka, 1954 Didemnum obscurum F. Monniot 1969 Didemnum perlucidum F. Monniot, 1983 Didemnum sp. Didemnum yolky C.Monniot & F. Monniot 1997 Diplosoma listerianum Milne Edwards, 1841 Order : Phlebobranchia Family : Ascidiidae Ascidia sp. Phallusia julinea Sluiter, 1915 Phallusia nigra Savigny, 1816 Order : Stolidobranchia Family : Styelidae Botryllus gregalis Sluiter, 1898 Botryllus niger Herdman, 1886 Botryllus sp. Eusynstyela cf. hartmeyeri Michaelsen, 1904 Polyandrocarpa sp. Styela canopus Savigny, 1816 Symplegma bahraini C.Monniot & F. Monniot 1997 Symplegma brakenhielmi Michaelsen, 1904 Family pyuridae Herdmania momus Savigny, 1816 Pyura curvigona Tokioka, 1950 Pyurid sp. Class Larvacea Larva Seymour Sewell Phylum Chordata Subphylum Urochordata * Class Larvacea Order Copelata Family Fritillariidae -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

Travels on the Backbone Crest (A Group of Embryonic Cells)
BOOKS & ARTS COMMENT EVOLUTION non-deuterostomes. In the most effective section, he strips vertebrates down to their parts, such as the nerve cord, notochord (fore- runner of the vertebral column) and neural Travels on the backbone crest (a group of embryonic cells). He even delves into the largely ignored gut and viscera. Chris Lowe lauds a study of vertebrate origins that Gee discusses how data from living and brings us up to date with a shifting field. fossilized hemichordates and echinoderms have facilitated the search for the origins of the defining chordate anatomies. He ome of the great remaining mysteries groups belong in the highlights how palaeontological, develop- in zoology concern origins — of multi- deuterostome lineage mental and genomic data now all support cellularity, complex nervous systems, of animals alongside the idea that the common ancestor of chor- Slife cycles and sex, for example. The chordates — have dates, hemichordates and echinoderms had evolutionary origin of vertebrates is among largely been resolved. pharyngeal gill slits for filter feeding. That the most intractable of these, despite more Others are as intrac- gives us a glimpse of the early chordate ances- than a century of work spanning a range of table as ever, including tor, which lived around 600 million years ago. disciplines and animal groups. where to place key fos- Other features, such as a complex brain, prob- In Across the Bridge, Henry Gee reviews sil groups such as the ably emerged much later. Having established the most recent research in this area. Gee curious vetulicolians, Across the Bridge: a hazy picture of the earliest chordates, Gee (the senior editor responsible for palae- which lived during Understanding focuses on building vertebrates and their the Origin of the ontology and evolutionary development the Cambrian period, Vertebrates defining features from the basic chordate at Nature) synthesizes contributions from some 541 million to HENRY GEE body plan, for example through spectacular anatomy, developmental biology, genomics, 485 million years ago. -

An Ontogenetic Switch Drives the Positive and Negative Selection of B Cells
An ontogenetic switch drives the positive and negative selection of B cells Xijin Xua, Mukta Deobagkar-Lelea, Katherine R. Bulla, Tanya L. Crockforda, Adam J. Meadb, Adam P. Cribbsc, David Simsc, Consuelo Anzilottia, and Richard J. Cornalla,1 aMedical Research Council Human Immunology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom; bMedical Research Council Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom; and cMedical Research Council, Weatherall Institute of Molecular Medicine, Centre for Computational Biology, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom Edited by Michael Reth, University of Freiburg, Freiburg, Germany, and approved January 6, 2020 (received for review September 3, 2019) + Developing B cells can be positively or negatively selected by self- BM HSCs increased CD5 B-1a B cell development (15), while antigens, but the mechanisms that determine these outcomes are expression of let-7b in FL pro-B cells blocked the development of incompletely understood. Here, we show that a B cell intrinsic B-1 B cells (17). These findings support the notion of hard-wired switch between positive and negative selection during ontogeny differences during ontogeny, but possibly downstream of the HSC is determined by a change from Lin28b to let-7 gene expression. commitment stage. Ectopic expression of a Lin28b transgene in murine B cells restored Several lines of evidence also suggest that B-1 B cells can un- the positive selection of autoreactive B-1 B cells by self-antigen in dergo positive selection, which is linked to their B cell receptor adult bone marrow. -

Sonic Hedgehog Signaling in Limb Development
REVIEW published: 28 February 2017 doi: 10.3389/fcell.2017.00014 Sonic Hedgehog Signaling in Limb Development Cheryll Tickle 1* and Matthew Towers 2* 1 Department of Biology and Biochemistry, University of Bath, Bath, UK, 2 Department of Biomedical Science, The Bateson Centre, University of Sheffield, Western Bank, Sheffield, UK The gene encoding the secreted protein Sonic hedgehog (Shh) is expressed in the polarizing region (or zone of polarizing activity), a small group of mesenchyme cells at the posterior margin of the vertebrate limb bud. Detailed analyses have revealed that Shh has the properties of the long sought after polarizing region morphogen that specifies positional values across the antero-posterior axis (e.g., thumb to little finger axis) of the limb. Shh has also been shown to control the width of the limb bud by stimulating mesenchyme cell proliferation and by regulating the antero-posterior length of the apical ectodermal ridge, the signaling region required for limb bud outgrowth and the laying down of structures along the proximo-distal axis (e.g., shoulder to digits axis) of the limb. It has been shown that Shh signaling can specify antero-posterior positional values in Edited by: limb buds in both a concentration- (paracrine) and time-dependent (autocrine) fashion. Andrea Erika Münsterberg, University of East Anglia, UK Currently there are several models for how Shh specifies positional values over time in the Reviewed by: limb buds of chick and mouse embryos and how this is integrated with growth. Extensive Megan Davey, work has elucidated downstream transcriptional targets of Shh signaling. Nevertheless, it University of Edinburgh, UK Robert Hill, remains unclear how antero-posterior positional values are encoded and then interpreted University of Edinburgh, UK to give the particular structure appropriate to that position, for example, the type of digit.