Rodent-Borne Bartonella Infection Varies According to Host Species Within and Among Cities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -

Bartonella Henselae Detected in Malignant Melanoma, a Preliminary Study
pathogens Article Bartonella henselae Detected in Malignant Melanoma, a Preliminary Study Marna E. Ericson 1, Edward B. Breitschwerdt 2 , Paul Reicherter 3, Cole Maxwell 4, Ricardo G. Maggi 2, Richard G. Melvin 5 , Azar H. Maluki 4,6 , Julie M. Bradley 2, Jennifer C. Miller 7, Glenn E. Simmons, Jr. 5 , Jamie Dencklau 4, Keaton Joppru 5, Jack Peterson 4, Will Bae 4, Janet Scanlon 4 and Lynne T. Bemis 5,* 1 T Lab Inc., 910 Clopper Road, Suite 220S, Gaithersburg, MD 20878, USA; [email protected] 2 Intracellular Pathogens Research Laboratory, Comparative Medicine Institute, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607, USA; [email protected] (E.B.B.); [email protected] (R.G.M.); [email protected] (J.M.B.) 3 Dermatology Clinic, Truman Medical Center, University of Missouri, Kansas City, MO 64108, USA; [email protected] 4 Department of Dermatology, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] (C.M.); [email protected] (A.H.M.); [email protected] (J.D.); [email protected] (J.P.); [email protected] (W.B.); [email protected] (J.S.) 5 Department of Biomedical Sciences, Duluth Campus, Medical School, University of Minnesota, Duluth, MN 55812, USA; [email protected] (R.G.M.); [email protected] (G.E.S.J.); [email protected] (K.J.) 6 Department of Dermatology, College of Medicine, University of Kufa, Kufa 54003, Iraq 7 Galaxy Diagnostics Inc., Research Triangle Park, NC 27709, USA; [email protected] Citation: Ericson, M.E.; * Correspondence: [email protected]; Tel.: +1-720-560-0278; Fax: +1-218-726-7906 Breitschwerdt, E.B.; Reicherter, P.; Maxwell, C.; Maggi, R.G.; Melvin, Abstract: Bartonella bacilliformis (B. -

(Rodentia: Ctenodactylidae) from the Miocene of Israel
RESEARCH ARTICLE A Transitional Gundi (Rodentia: Ctenodactylidae) from the Miocene of Israel Raquel López-Antoñanzas1,2*, Vitaly Gutkin3, Rivka Rabinovich4, Ran Calvo5, Aryeh Grossman6,7 1 School of Earth Sciences, University of Bristol, Bristol, United Kingdom, 2 Departamento de Paleobiología, Museo Nacional de Ciencias Naturales-CSIC, Madrid, Spain, 3 The Harvey M. Krueger Family Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, Israel, 4 National Natural History Collections, Institute of Earth Sciences and Institute of Archaeology, The Hebrew University of Jerusalem, Jerusalem, Israel, 5 Geological Survey of Israel, Jerusalem, Israel, 6 Arizona College of Osteopathic Medicine, Midwestern University, Glendale, AZ, United States of America, 7 School of Human Evolution and Social Change, Arizona State University, Tempe, AZ, United States of America * [email protected] Abstract OPEN ACCESS We describe a new species of gundi (Rodentia: Ctenodactylidae: Ctenodactylinae), Sayi- mys negevensis, on the basis of cheek teeth from the Early Miocene of the Rotem Basin, Citation: López-Antoñanzas R, Gutkin V, Rabinovich R, Calvo R, Grossman A (2016) A Transitional Gundi southern Israel. The Rotem ctenodactylid differs from all known ctenodactylid species, (Rodentia: Ctenodactylidae) from the Miocene of including Sayimys intermedius, which was first described from the Middle Miocene of Saudi Israel. PLoS ONE 11(4): e0151804. doi:10.1371/ Arabia. Instead, it most resembles Sayimys baskini from the Early Miocene of Pakistan in journal.pone.0151804 characters of the m1-2 (e.g., the mesoflexid shorter than the metaflexid, the obliquely orien- Editor: Laurent Viriot, Team 'Evolution of Vertebrate tated hypolophid, and the presence of a strong posterolabial ledge) and the upper molars Dentition', FRANCE (e.g., the paraflexus that is longer than the metaflexus). -

Multiple Molecular Evidences for a Living Mammalian Fossil
Multiple molecular evidences for a living mammalian fossil Dorothe´ e Huchon†‡, Pascale Chevret§¶, Ursula Jordanʈ, C. William Kilpatrick††, Vincent Ranwez§, Paulina D. Jenkins‡‡, Ju¨ rgen Brosiusʈ, and Ju¨ rgen Schmitz‡ʈ †Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv 69978, Israel; §Department of Paleontology, Phylogeny, and Paleobiology, Institut des Sciences de l’Evolution, cc064, Universite´Montpellier II, Place E. Bataillon, 34095 Montpellier Cedex 5, France; ʈInstitute of Experimental Pathology, University of Mu¨nster, D-48149 Mu¨nster, Germany; ††Department of Biology, University of Vermont, Burlington, VT 05405-0086; and ‡‡Department of Zoology, The Natural History Museum, London SW7 5BD, United Kingdom Edited by Francisco J. Ayala, University of California, Irvine, CA, and approved March 18, 2007 (received for review February 11, 2007) Laonastes aenigmamus is an enigmatic rodent first described in their classification as a diatomyid suggests that Laonastes is a 2005. Molecular and morphological data suggested that it is the living fossil and a ‘‘Lazarus taxon.’’ sole representative of a new mammalian family, the Laonastidae, The two research teams also disagreed on the taxonomic and a member of the Hystricognathi. However, the validity of this position of Laonastes. According to Jenkins et al. (2), Laonastes family is controversial because fossil-based phylogenetic analyses is either the most basal group of the hystricognaths (Fig. 2A)or suggest that Laonastes is a surviving member of the Diatomyidae, nested within the hystricognaths (Fig. 2B). According to Dawson a family considered to have been extinct for 11 million years. et al. (3), Laonastes and the other Diatomyidae are the sister According to these data, Laonastes and Diatomyidae are the sister clade of the family Ctenodactylidae (i.e., gundies), a family that clade of extant Ctenodactylidae (i.e., gundies) and do not belong does not belong to the Hystricognathi, but to which it is to the Hystricognathi. -
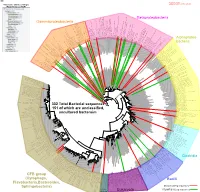
Bacteria Clostridia Bacilli Eukaryota CFB Group
AM935842.1.1361 uncultured Burkholderiales bacterium Class Betaproteobacteria AY283260.1.1552 Alcaligenes sp. PCNB−2 Class Betaproteobacteria AM934953.1.1374 uncultured Burkholderiales bacterium Class Betaproteobacteria AJ581593.1.1460 uncultured betaAM936569.1.1351 proteobacterium uncultured Class Betaproteobacteria Derxia sp. Class Betaproteobacteria AJ581621.1.1418 uncultured beta proteobacterium Class Betaproteobacteria DQ248272.1.1498 uncultured soil bacterium soil uncultured DQ248272.1.1498 DQ248235.1.1498 uncultured soil bacterium RS49 DQ248270.1.1496 uncultured soil bacterium DQ256489.1.1211 Variovorax paradoxus Class Betaproteobacteria Class paradoxus Variovorax DQ256489.1.1211 AF523053.1.1486 uncultured Comamonadaceae bacterium Class Betaproteobacteria AY706442.1.1396 uncultured bacterium uncultured AY706442.1.1396 AJ536763.1.1422 uncultured bacterium CS000359.1.1530 Variovorax paradoxus Class Betaproteobacteria Class paradoxus Variovorax CS000359.1.1530 AY168733.1.1411 uncultured bacterium AJ009470.1.1526 uncultured bacterium SJA−62 Class Betaproteobacteria Class SJA−62 bacterium uncultured AJ009470.1.1526 AY212561.1.1433 uncultured bacterium D16212.1.1457 Rhodoferax fermentans Class Betaproteobacteria Class fermentans Rhodoferax D16212.1.1457 AY957894.1.1546 uncultured bacterium AJ581620.1.1452 uncultured beta proteobacterium Class Betaproteobacteria RS76 AY625146.1.1498 uncultured bacterium RS65 DQ316832.1.1269 uncultured beta proteobacterium Class Betaproteobacteria DQ404909.1.1513 uncultured bacterium uncultured DQ404909.1.1513 AB021341.1.1466 bacterium rM6 AJ487020.1.1500 uncultured bacterium uncultured AJ487020.1.1500 RS7 RS86RC AF364862.1.1425 bacterium BA128 Class Betaproteobacteria AY957931.1.1529 uncultured bacterium uncultured AY957931.1.1529 CP000884.723807.725332 Delftia acidovorans SPH−1 Class Betaproteobacteria AY957923.1.1520 uncultured bacterium uncultured AY957923.1.1520 RS18 AY957918.1.1527 uncultured bacterium uncultured AY957918.1.1527 AY945883.1.1500 uncultured bacterium AF526940.1.1489 uncultured Ralstonia sp. -

Spatial Variation in the Parasite Communities and Genomic Structure of Urban Rats in New York City
Received: 26 March 2017 DOI: 10.1111/zph.12418 ORIGINAL ARTICLE Spatial variation in the parasite communities and genomic structure of urban rats in New York City L. P. Angley1 | M. Combs2 | C. Firth3,4 | M. J. Frye5 | I. Lipkin3 | J. L. Richardson1 | J. Munshi-South2 1Department of Biology, Providence College, Providence, RI, USA Summary 2Louis Calder Center and Department of Brown rats (Rattus norvegicus) are a globally distributed pest. Urban habitats can sup- Biological Sciences, Fordham University, port large infestations of rats, posing a potential risk to public health from the para- Armonk, NY, USA sites and pathogens they carry. Despite the potential influence of rodent- borne 3Mailman School of Public Health, Columbia University, New York, NY, USA zoonotic diseases on human health, it is unclear how urban habitats affect the struc- 4School of BioSciences, The University of ture and transmission dynamics of ectoparasite and microbial communities (all Melbourne, Parkville, VIC, Australia referred to as “parasites” hereafter) among rat colonies. In this study, we use ecologi- 5New York State Integrated Pest Management Program, Cornell University, Geneva, NY, USA cal data on parasites and genomic sequencing of their rat hosts to examine associa- tions between spatial proximity, genetic relatedness and the parasite communities Correspondence Jonathan L. Richardson, Department of associated with 133 rats at five sites in sections of New York City with persistent rat Biology, Providence College, Providence, RI, infestations. We build on previous work showing that rats in New York carry a wide USA. Email: [email protected] variety of parasites and report that these communities differ significantly among sites, even across small geographical distances. -

Isolation of Francisella Tularensis from Skin Ulcer After a Tick Bite, Austria, 2020
microorganisms Case Report Isolation of Francisella tularensis from Skin Ulcer after a Tick Bite, Austria, 2020 Mateusz Markowicz 1,*, Anna-Margarita Schötta 1 , Freya Penatzer 2, Christoph Matscheko 2, Gerold Stanek 1, Hannes Stockinger 1 and Josef Riedler 2 1 Center for Pathophysiology, Infectiology and Immunology, Institute for Hygiene and Applied Immunology, Medical University of Vienna, Kinderspitalgasse 15, A-1090 Vienna, Austria; [email protected] (A.-M.S.); [email protected] (G.S.); [email protected] (H.S.) 2 Kardinal Schwarzenberg Klinikum, Kardinal Schwarzenbergplatz 1, A-5620 Schwarzach, Austria; [email protected] (F.P.); [email protected] (C.M.); [email protected] (J.R.) * Correspondence: [email protected]; Tel.: +43-1-40160-33023 Abstract: Ulceroglandular tularemia is caused by the transmission of Francisella tularensis by arthro- pods to a human host. We report a case of tick-borne tularemia in Austria which was followed by an abscess formation in a lymph node, making drainage necessary. F. tularensis subsp. holarctica was identified by PCR and multilocus sequence typing. Keywords: tularemia; Francisella tularensis; tick; multi locus sequence typing Depending on the transmission route of Francisella tularensis, tularemia can present Citation: Markowicz, M.; Schötta, as a local infection or a systemic disease [1]. Transmission of the pathogen takes place A.-M.; Penatzer, F.; Matscheko, C.; by contact with infected animals, by bites of arthropods or through contaminated water Stanek, G.; Stockinger, H.; Riedler, J. and soil. Hares and wild rabbits are the main reservoirs of the pathogen in Austria [2]. -

Emerging Bartonellosis Christoph Dehio & Anna Sander
Emerging bartonellosis Christoph Dehio & Anna Sander Bartonellae are arthropod-borne pathogens of they cause a long-lasting infection within the red blood Ggrowing medical importance. Until the early cells (intraerythrocytic bacteraemia). Blood-sucking 1990s, only two species of this bacterial genus, arthropod vectors transmit the bacteria from this reservoir B. bacilliformis and B. quintana, were recognized as caus- to new hosts. Incidental infection of non-reservoir hosts ing disease in humans. In addition to re-emergence of the (e.g. humans by the zoonotic species) may cause disease, human-specific B. quintana, a number of zoonotic but does not result in intraerythrocytic infection. Bartonella species have now been recognized as causative agents for a broadening spectrum of diseases that can be Natural history and epidemiology transmitted to humans from their animal hosts. Most Humans are the only known reservoir for two Bartonella prominently, B. henselae is an important zoonotic species, B. bacilliformis and B. quintana. pathogen that is frequently passed from its feline B. quintana was a leading cause of infectious morbidity reservoir to humans. among soldiers during World War I, and recurred on the Bacteria of the genus Bartonella are Gram-negative, East European front in World War II. The disease, pleomorphic, fastidious bacilli that belong to the α-2 Trench fever, is rarely fatal and is characterized by an subclass of Proteobacteria. All Bartonella species appear intraerythrocytic bacteraemia with recurrent, cycling to have a specific mammalian species as a host, in which fever. It is transmitted among humans by the human body louse Pediculus humanus. Although almost forgotten Table 1. -

Gridlock and Beltways: the Genetic Context of Urban Invasions
Oecologia (2020) 192:615–628 https://doi.org/10.1007/s00442-020-04614-y CONCEPTS, REVIEWS AND SYNTHESES Gridlock and beltways: the genetic context of urban invasions E. M. X. Reed1 · M. E. Serr1 · A. S. Maurer1 · M. O. Burford Reiskind1 Received: 8 May 2019 / Accepted: 30 January 2020 / Published online: 13 February 2020 © Springer-Verlag GmbH Germany, part of Springer Nature 2020 Abstract The rapid expansion of urban land across the globe presents new and numerous opportunities for invasive species to spread and fourish. Ecologists historically rejected urban ecosystems as important environments for ecology and evolution research but are beginning to recognize the importance of these systems in shaping the biology of invasion. Urbanization can aid the introduction, establishment, and spread of invaders, and these processes have substantial consequences on native species and ecosystems. Therefore, it is valuable to understand how urban areas infuence populations at all stages in the invasion process. Population genetic tools are essential to explore the driving forces of invasive species dispersal, connectivity, and adaptation within cities. In this review, we synthesize current research about the infuence of urban landscapes on invasion genetics dynamics. We conclude that urban areas are not only points of entry for many invasive species, they also facilitate population establishment, are pools for genetic diversity, and provide corridors for further spread both within and out of cities. We recommend the continued use of genetic studies to inform invasive species management and to understand the underlying ecological and evolutionary processes governing successful invasion. Keywords Synthesis · Population genetics · Landscape genetics · Invasive species · Urban ecosystems Introduction increasing extent of urban land cover and the synergistic relationship between urbanization and invasion (Seto et al. -

Bartonella Henselae
Maggi et al. Parasites & Vectors 2013, 6:101 http://www.parasitesandvectors.com/content/6/1/101 RESEARCH Open Access Bartonella henselae bacteremia in a mother and son potentially associated with tick exposure Ricardo G Maggi1,3*, Marna Ericson2, Patricia E Mascarelli1, Julie M Bradley1 and Edward B Breitschwerdt1 Abstract Background: Bartonella henselae is a zoonotic, alpha Proteobacterium, historically associated with cat scratch disease (CSD), but more recently associated with persistent bacteremia, fever of unknown origin, arthritic and neurological disorders, and bacillary angiomatosis, and peliosis hepatis in immunocompromised patients. A family from the Netherlands contacted our laboratory requesting to be included in a research study (NCSU-IRB#1960), designed to characterize Bartonella spp. bacteremia in people with extensive arthropod or animal exposure. All four family members had been exposed to tick bites in Zeeland, southwestern Netherlands. The mother and son were exhibiting symptoms including fatigue, headaches, memory loss, disorientation, peripheral neuropathic pain, striae (son only), and loss of coordination, whereas the father and daughter were healthy. Methods: Each family member was tested for serological evidence of Bartonella exposure using B. vinsonii subsp. berkhoffii genotypes I-III, B. henselae and B. koehlerae indirect fluorescent antibody assays and for bacteremia using the BAPGM enrichment blood culture platform. Results: The mother was seroreactive to multiple Bartonella spp. antigens and bacteremia was confirmed by PCR amplification of B. henselae DNA from blood, and from a BAPGM blood agar plate subculture isolate. The son was not seroreactive to any Bartonella sp. antigen, but B. henselae DNA was amplified from several blood and serum samples, from BAPGM enrichment blood culture, and from a cutaneous striae biopsy. -

Palaeogene Fossil Record, Phylogeny, Dental Evolution and Historical Biogeography Laurent Marivaux, Myriam Boivin
Emergence of hystricognathous rodents: Palaeogene fossil record, phylogeny, dental evolution and historical biogeography Laurent Marivaux, Myriam Boivin To cite this version: Laurent Marivaux, Myriam Boivin. Emergence of hystricognathous rodents: Palaeogene fossil record, phylogeny, dental evolution and historical biogeography. Zoological Journal of the Linnean Society, Linnean Society of London, 2019, 187 (3), pp.929-964. 10.1093/zoolinnean/zlz048. hal-02263901 HAL Id: hal-02263901 https://hal.umontpellier.fr/hal-02263901 Submitted on 1 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Zoological Journal of the Linnean Society Emergence of hystricognathous rodents: Palaeogene fossil record, phylogeny, dental evolution, and historical biogeography Journal: Zoological Journal of the Linnean Society Manuscript ID ZOJ-12-2018-3565.R2 Manuscript Type:ForOriginal Review Article Only Africa < Geography, Asia < Geography, mandible < Anatomy, dental Keywords: evolution < Evolution, Eocene < Palaeontology, Oligocene < Palaeontology Although phylogenies imply Asia as the ancestral homeland of the Hystricognathi clade, curiously the oldest known fossil occurrences are not from Asia but from Africa and South America, where they appear by the late middle Eocene. Here we performed cladistic and Bayesian assessments of the dental evidence documenting early ctenohystricans, including several Asian “ctenodactyloids”, all Palaeogene Asian and African hystricognaths, and two early South American hystricognaths. -

Bartonella: Feline Diseases and Emerging Zoonosis
BARTONELLA: FELINE DISEASES AND EMERGING ZOONOSIS WILLIAM D. HARDY, JR., V.M.D. Director National Veterinary Laboratory, Inc. P.O Box 239 Franklin Lakes, New Jersey 07417 201-891-2992 www.natvetlab.com or .net Gingivitis Proliferative Gingivitis Conjunctivitis/Blepharitis Uveitis & Conjunctivitis URI Oral Ulcers Stomatitis Lymphadenopathy TABLE OF CONTENTS Page SUMMARY……………………………………………………………………………………... i INTRODUCTION……………………………………………………………………………… 1 MICROBIOLOGY……………………………………………………………………………... 1 METHODS OF DETECTION OF BARTONELLA INFECTION.………………………….. 1 Isolation from Blood…………………………………………………………………….. 2 Serologic Tests…………………………………………………………………………… 2 SEROLOGY……………………………………………………………………………………… 3 CATS: PREVALENCE OF BARTONELLA INFECTIONS…………………………………… 4 Geographic Risk factors for Infection……………………………………………………. 5 Risk Factors for Infection………………………………………………………………… 5 FELINE BARTONELLA DISEASES………………………………………………………….… 6 Bartonella Pathogenesis………………………………………………………………… 7 Therapy of Feline Bartonella Diseases…………………………………………………… 14 Clinical Therapy Results…………………………………………………………………. 15 DOGS: PREVALENCE OF BARTONELLA INFECTIONS…………………………………. 17 CANINE BARTONELLA DISEASES…………………………………………………………... 17 HUMAN BARTONELLA DISEASES…………………………………………………………… 18 Zoonotic Case Study……………………………………………………………………... 21 FELINE BLOOD DONORS……………………………………………………………………. 21 REFERENCES………………………………………………………………………………….. 22 This work was initiated while Dr. Hardy was: Professor of Medicine, Albert Einstein College of Medicine of Yeshiva University, Bronx, New York and Director,