Bartonellosis Ed Breitschwerdt, DVM (Ed [email protected]) Tests Available at VBDDL: Serology (IFA) and PCR on Blood Or CSF
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parinaud's Oculoglandular Syndrome
Tropical Medicine and Infectious Disease Case Report Parinaud’s Oculoglandular Syndrome: A Case in an Adult with Flea-Borne Typhus and a Review M. Kevin Dixon 1, Christopher L. Dayton 2 and Gregory M. Anstead 3,4,* 1 Baylor Scott & White Clinic, 800 Scott & White Drive, College Station, TX 77845, USA; [email protected] 2 Division of Critical Care, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA; [email protected] 3 Medical Service, South Texas Veterans Health Care System, San Antonio, TX 78229, USA 4 Division of Infectious Diseases, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA * Correspondence: [email protected]; Tel.: +1-210-567-4666; Fax: +1-210-567-4670 Received: 7 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Parinaud’s oculoglandular syndrome (POGS) is defined as unilateral granulomatous conjunctivitis and facial lymphadenopathy. The aims of the current study are to describe a case of POGS with uveitis due to flea-borne typhus (FBT) and to present a diagnostic and therapeutic approach to POGS. The patient, a 38-year old man, presented with persistent unilateral eye pain, fever, rash, preauricular and submandibular lymphadenopathy, and laboratory findings of FBT: hyponatremia, elevated transaminase and lactate dehydrogenase levels, thrombocytopenia, and hypoalbuminemia. His condition rapidly improved after starting doxycycline. Soon after hospitalization, he was diagnosed with uveitis, which responded to topical prednisolone. To derive a diagnostic and empiric therapeutic approach to POGS, we reviewed the cases of POGS from its various causes since 1976 to discern epidemiologic clues and determine successful diagnostic techniques and therapies; we found multiple cases due to cat scratch disease (CSD; due to Bartonella henselae) (twelve), tularemia (ten), sporotrichosis (three), Rickettsia conorii (three), R. -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -

Abstract Pultorak, Elizabeth Lauren
ABSTRACT PULTORAK, ELIZABETH LAUREN. The Epidemiology of Lyme Disease and Bartonellosis in Humans and Animals. (Under the direction of Edward B. Breitschwerdt). The expansion of vector borne diseases in humans, a variety of mammalian hosts, and arthropod vectors draws attention to the need for enhanced diagnostic techniques for documenting infection in hosts, effective vector control, and treatment of individuals with associated diseases. Through improved diagnosis of vector-borne disease in both humans and animals, epidemiological studies to elucidate clinical associations or spatio-temporal relationships can be assessed. Veterinarians, through the use of the C6 peptide in the SNAP DX test kit, may be able to evaluate the changing epidemiology of borreliosis through their canine population. We developed a survey to evaluate the practices and perceptions of veterinarians in North Carolina regarding borreliosis in dogs across different geographic regions of the state. We found that veterinarians’ perception of the risk of borreliosis in North Carolina was consistent with recent scientific reports pertaining to geographic expansion of borreliosis in the state. Veterinarians should promote routine screening of dogs for Borrelia burgdorferi exposure as a simple, inexpensive form of surveillance in this transitional geographic region. We next conducted two separate studies to evaluate Bartonella spp. bacteremia or presence of antibodies against B. henselae, B. koehlerae, or B. vinsonii subsp. berkhoffii in 296 patients examined by a rheumatologist and 192 patients with animal exposure (100%) and recent animal bites and scratches (88.0%). Among 296 patients examined by a rheumatologist, prevalence of antibodies (185 [62%]) and Bartonella spp. bacteremia (122 [41.1%]) was high. -

CASE REPORT the PATIENT 33-Year-Old Woman
CASE REPORT THE PATIENT 33-year-old woman SIGNS & SYMPTOMS – 6-day history of fever Katherine Lazet, DO; – Groin pain and swelling Stephanie Rutterbush, MD – Recent hiking trip in St. Vincent Ascension Colorado Health, Evansville, Ind (Dr. Lazet); Munson Healthcare Ostego Memorial Hospital, Lewiston, Mich (Dr. Rutterbush) [email protected] The authors reported no potential conflict of interest THE CASE relevant to this article. A 33-year-old Caucasian woman presented to the emergency department with a 6-day his- tory of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado. Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high. The patient’s work-up included a nega- tive pregnancy test and urinalysis. A com- FIGURE 1 plete blood count demonstrated a white CT scan from admission blood cell count of 8.6 (4.3-10.5) × 103/UL was revealing with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). -

Bartonella: Emerging Pathogen Or Emerging Awareness?
International Journal of Infectious Diseases (2009) 13, 3—8 http://intl.elsevierhealth.com/journals/ijid PERSPECTIVE Bartonella: emerging pathogen or emerging awareness? Elin Mogollon-Pasapera, Laszlo Otvos Jr, Antonio Giordano, Marco Cassone * Sbarro Health Research Organization, College of Science and Technology, Temple University, BioLifeScience Building suite 419, 1900 N 12th Street, 19122 Philadelphia, PA, USA Received 17 October 2007; received in revised form 26 January 2008; accepted 14 April 2008 Corresponding Editor: William Cameron, Ottawa, Canada KEYWORDS Summary The number of known Bartonella species is rapidly growing. Some of them are Bartonella; responsible for distinct infectious diseases and show different prevalence and antibiotic suscept- Carrion’s disease; ibility profiles. Not only have some vectors of Bartonella not been fully characterized, but also Epidemiology; intermediate hosts are actually much more numerous and diverse than previously thought. Among Cat-scratch disease; these, dogs differ from cats because they tend to suffer an overt disease similar to humans, thus Therapy providing the base for a useful animal indicator and research model. Among the debilitating conditions with an unclear impact on the course of these infections, specific conditions (e.g., homelessness, alcoholism) have been linked to a much higher prevalence and to high risk of unfavorable outcome. Due to the limited arsenal of antibiotics effective in vivo on this peculiar intracellular pathogen, the risk/benefit balance of antibiotic therapy is sometimes difficult to draw. In this evolving picture, the recent discoveries of new species highlights the importance of basic molecular biology resources that would bring major public health benefits if available in endemic areas, and specifically in many areas of Peru and Bolivia. -

Bartonella Henselae Detected in Malignant Melanoma, a Preliminary Study
pathogens Article Bartonella henselae Detected in Malignant Melanoma, a Preliminary Study Marna E. Ericson 1, Edward B. Breitschwerdt 2 , Paul Reicherter 3, Cole Maxwell 4, Ricardo G. Maggi 2, Richard G. Melvin 5 , Azar H. Maluki 4,6 , Julie M. Bradley 2, Jennifer C. Miller 7, Glenn E. Simmons, Jr. 5 , Jamie Dencklau 4, Keaton Joppru 5, Jack Peterson 4, Will Bae 4, Janet Scanlon 4 and Lynne T. Bemis 5,* 1 T Lab Inc., 910 Clopper Road, Suite 220S, Gaithersburg, MD 20878, USA; [email protected] 2 Intracellular Pathogens Research Laboratory, Comparative Medicine Institute, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607, USA; [email protected] (E.B.B.); [email protected] (R.G.M.); [email protected] (J.M.B.) 3 Dermatology Clinic, Truman Medical Center, University of Missouri, Kansas City, MO 64108, USA; [email protected] 4 Department of Dermatology, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] (C.M.); [email protected] (A.H.M.); [email protected] (J.D.); [email protected] (J.P.); [email protected] (W.B.); [email protected] (J.S.) 5 Department of Biomedical Sciences, Duluth Campus, Medical School, University of Minnesota, Duluth, MN 55812, USA; [email protected] (R.G.M.); [email protected] (G.E.S.J.); [email protected] (K.J.) 6 Department of Dermatology, College of Medicine, University of Kufa, Kufa 54003, Iraq 7 Galaxy Diagnostics Inc., Research Triangle Park, NC 27709, USA; [email protected] Citation: Ericson, M.E.; * Correspondence: [email protected]; Tel.: +1-720-560-0278; Fax: +1-218-726-7906 Breitschwerdt, E.B.; Reicherter, P.; Maxwell, C.; Maggi, R.G.; Melvin, Abstract: Bartonella bacilliformis (B. -

Protecting Yourself from Zoonotic Infection
PROTECTING YOURSELF FROM ZOONOTIC INFECTION What is a Zoonoses? A zoonotic disease is an infection that is naturally transmitted from vertebrate animals to human beings. Many of these infections are transmitted directly but others are passed via vectors such as mosquitoes or fleas. Not all animal diseases are zoonotic and far more human illnesses result from contact with other humans than from animals. However, it is important for anyone working with animals to be aware of the potential for infection and takes steps to prevent exposure. The following lists some of the more common or severe zoonoses and ways to help protect yourself and others from exposure. IMPORTANT RULES TO HELP YOU AVOID DEVELOPING A SERIOUS ZOONOTIC ILLNESS: Stay current on appropriate vaccinations, such as tetanus and rabies. Wash your hands frequently with antibacterial soap, especially after handling any animal and prior to eating or smoking. Wear long pants and sturdy shoes or boots. Use gloves when handling animals and when cleaning up feces, urine, or vomit. Immediately disinfect scratches and bite wounds thoroughly. Keep scratches or other abrasions covered, especially when cleaning up after animals. Learn safe and humane animal-handling techniques and use proper equipment. Seek assistance when handling animals whose dispositions are questionable. If exposed to tick-infested areas, check your body and clothing frequently. Use tweezers and wear gloves to remove ticks, taking care not to squeeze or puncture the body of the tick. Report any bites or injuries to a supervisor and seek medical treatment as appropriate. Tell your physician that you work closely with animals, and visit him or her regularly. -
Vectra® for Cats & Kittens and Vectra® for Cats
This is love. Wrapped in fur. Protect the love. FLEA CONTROL Facts About Fleas Can jump up to Can lay eggs 13” 24 hours after biting Females can lay 50 eggs per day Transmit and cause diseases like: Flea Allergy Dermatitis Tapeworm disease Bartonella Life cycle can be completed in Anemia (severe infestations) Feline Infectious Anemia 3 weeks Bubonic Plague Why are flea infestations so hard to get rid of? ONLY 5% OF THE FLEAS IN YOUR CAT’S ENVIRONMENT ARE ADULTS THAT BITE YOUR CAT. The remaining 95% are in the development stage ready to become adults within 3 weeks. 5% Adults 35% Larvae 50% Eggs 10% Pupae Vectra® for Cats & Kittens and Vectra® for Cats Vectra® for Cats & Kittens AND Vectra® for Cats protect your favorite feline from all stages of fleas. Applying Vectra® every month helps prevent flea infestation, re-infestation, and protects your cat or kitten from flea-borne diseases. Fast-Acting • Kills through contact so fleas do not have to bite in order to die • Protects cats and kittens against flea-borne diseases, including tularemia, rickettsiosis, Life cycle can be completed in bartonellosis, and tapeworm Flea Protection • Kills fleas at all life stages for 1 month • Adults • Eggs • Larvae • Pupae • Remains effective after exposure to sunlight Convenient • Patented applicator makes application easy, clean, and accurate • Can be used on kittens as young as 8 weeks of age How to apply Vectra® for Cats & Kittens and Vectra® for Cats Before administering Vectra® for the first time, you should ask your veterinarian to demonstrate the proper application technique. -
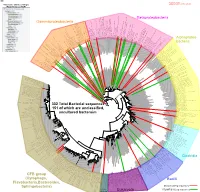
Bacteria Clostridia Bacilli Eukaryota CFB Group
AM935842.1.1361 uncultured Burkholderiales bacterium Class Betaproteobacteria AY283260.1.1552 Alcaligenes sp. PCNB−2 Class Betaproteobacteria AM934953.1.1374 uncultured Burkholderiales bacterium Class Betaproteobacteria AJ581593.1.1460 uncultured betaAM936569.1.1351 proteobacterium uncultured Class Betaproteobacteria Derxia sp. Class Betaproteobacteria AJ581621.1.1418 uncultured beta proteobacterium Class Betaproteobacteria DQ248272.1.1498 uncultured soil bacterium soil uncultured DQ248272.1.1498 DQ248235.1.1498 uncultured soil bacterium RS49 DQ248270.1.1496 uncultured soil bacterium DQ256489.1.1211 Variovorax paradoxus Class Betaproteobacteria Class paradoxus Variovorax DQ256489.1.1211 AF523053.1.1486 uncultured Comamonadaceae bacterium Class Betaproteobacteria AY706442.1.1396 uncultured bacterium uncultured AY706442.1.1396 AJ536763.1.1422 uncultured bacterium CS000359.1.1530 Variovorax paradoxus Class Betaproteobacteria Class paradoxus Variovorax CS000359.1.1530 AY168733.1.1411 uncultured bacterium AJ009470.1.1526 uncultured bacterium SJA−62 Class Betaproteobacteria Class SJA−62 bacterium uncultured AJ009470.1.1526 AY212561.1.1433 uncultured bacterium D16212.1.1457 Rhodoferax fermentans Class Betaproteobacteria Class fermentans Rhodoferax D16212.1.1457 AY957894.1.1546 uncultured bacterium AJ581620.1.1452 uncultured beta proteobacterium Class Betaproteobacteria RS76 AY625146.1.1498 uncultured bacterium RS65 DQ316832.1.1269 uncultured beta proteobacterium Class Betaproteobacteria DQ404909.1.1513 uncultured bacterium uncultured DQ404909.1.1513 AB021341.1.1466 bacterium rM6 AJ487020.1.1500 uncultured bacterium uncultured AJ487020.1.1500 RS7 RS86RC AF364862.1.1425 bacterium BA128 Class Betaproteobacteria AY957931.1.1529 uncultured bacterium uncultured AY957931.1.1529 CP000884.723807.725332 Delftia acidovorans SPH−1 Class Betaproteobacteria AY957923.1.1520 uncultured bacterium uncultured AY957923.1.1520 RS18 AY957918.1.1527 uncultured bacterium uncultured AY957918.1.1527 AY945883.1.1500 uncultured bacterium AF526940.1.1489 uncultured Ralstonia sp. -

Isolation of Francisella Tularensis from Skin Ulcer After a Tick Bite, Austria, 2020
microorganisms Case Report Isolation of Francisella tularensis from Skin Ulcer after a Tick Bite, Austria, 2020 Mateusz Markowicz 1,*, Anna-Margarita Schötta 1 , Freya Penatzer 2, Christoph Matscheko 2, Gerold Stanek 1, Hannes Stockinger 1 and Josef Riedler 2 1 Center for Pathophysiology, Infectiology and Immunology, Institute for Hygiene and Applied Immunology, Medical University of Vienna, Kinderspitalgasse 15, A-1090 Vienna, Austria; [email protected] (A.-M.S.); [email protected] (G.S.); [email protected] (H.S.) 2 Kardinal Schwarzenberg Klinikum, Kardinal Schwarzenbergplatz 1, A-5620 Schwarzach, Austria; [email protected] (F.P.); [email protected] (C.M.); [email protected] (J.R.) * Correspondence: [email protected]; Tel.: +43-1-40160-33023 Abstract: Ulceroglandular tularemia is caused by the transmission of Francisella tularensis by arthro- pods to a human host. We report a case of tick-borne tularemia in Austria which was followed by an abscess formation in a lymph node, making drainage necessary. F. tularensis subsp. holarctica was identified by PCR and multilocus sequence typing. Keywords: tularemia; Francisella tularensis; tick; multi locus sequence typing Depending on the transmission route of Francisella tularensis, tularemia can present Citation: Markowicz, M.; Schötta, as a local infection or a systemic disease [1]. Transmission of the pathogen takes place A.-M.; Penatzer, F.; Matscheko, C.; by contact with infected animals, by bites of arthropods or through contaminated water Stanek, G.; Stockinger, H.; Riedler, J. and soil. Hares and wild rabbits are the main reservoirs of the pathogen in Austria [2]. -

Emerging Bartonellosis Christoph Dehio & Anna Sander
Emerging bartonellosis Christoph Dehio & Anna Sander Bartonellae are arthropod-borne pathogens of they cause a long-lasting infection within the red blood Ggrowing medical importance. Until the early cells (intraerythrocytic bacteraemia). Blood-sucking 1990s, only two species of this bacterial genus, arthropod vectors transmit the bacteria from this reservoir B. bacilliformis and B. quintana, were recognized as caus- to new hosts. Incidental infection of non-reservoir hosts ing disease in humans. In addition to re-emergence of the (e.g. humans by the zoonotic species) may cause disease, human-specific B. quintana, a number of zoonotic but does not result in intraerythrocytic infection. Bartonella species have now been recognized as causative agents for a broadening spectrum of diseases that can be Natural history and epidemiology transmitted to humans from their animal hosts. Most Humans are the only known reservoir for two Bartonella prominently, B. henselae is an important zoonotic species, B. bacilliformis and B. quintana. pathogen that is frequently passed from its feline B. quintana was a leading cause of infectious morbidity reservoir to humans. among soldiers during World War I, and recurred on the Bacteria of the genus Bartonella are Gram-negative, East European front in World War II. The disease, pleomorphic, fastidious bacilli that belong to the α-2 Trench fever, is rarely fatal and is characterized by an subclass of Proteobacteria. All Bartonella species appear intraerythrocytic bacteraemia with recurrent, cycling to have a specific mammalian species as a host, in which fever. It is transmitted among humans by the human body louse Pediculus humanus. Although almost forgotten Table 1. -

61% of All Human Pathogens Are Zoonotic (Passed from Animals to Humans), and Many Are Transmitted Through Inhaling Dust Particles Or Contact with Animal Wastes
Zoonotic Diseases Fast Facts: 61% of all human pathogens are zoonotic (passed from animals to humans), and many are transmitted through inhaling dust particles or contact with animal wastes. Some of the diseases we can get from our pets may be fatal if they go undetected or undiagnosed. All are serious threats to human health, but can usually be avoided by observing a few precautions, the most effective of which is washing your hands after touching animals or their wastes. Regular visits to the veterinarian for prevention, diagnosis, and treatment of zoonotic diseases will help limit disease in your pet. Source: http://www.cdc.gov/healthypets/ Some common zoonotic diseases humans can get through their pets: Zoonotic Disease & its Effect on How Contact is Made Humans Bartonellosis (cat scratch disease) – an Bartonella bacteria are transferred to humans through infection from the bacteria Bartonella a bite or scratch. Do not play with stray cats, and henselae that causes fever and swollen keep your cat free of fleas. Always wash hands after lymph nodes. handling your cat. Capnocytophaga infection – an Capnocytophaga canimorsus is the main human infection caused by bacteria that can pathogen associated with being licked or bitten by an develop into septicemia, meningitis, infected dog and may present a problem for those and endocarditis. who are immunosuppressed. Cellulitis – a disease occurring when Bacterial organisms from the Pasteurella species live bacteria such as Pasteurella multocida in the mouths of most cats, as well as a significant cause a potentially serious infection of number of dogs and other animals. These bacteria the skin.