Annotated List of Mimallonidae (Lepidoptera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Butterflies of Ontario & Summaries of Lepidoptera
ISBN #: 0-921631-12-X BUTTERFLIES OF ONTARIO & SUMMARIES OF LEPIDOPTERA ENCOUNTERED IN ONTARIO IN 1991 BY A.J. HANKS &Q.F. HESS PRODUCTION BY ALAN J. HANKS APRIL 1992 CONTENTS 1. INTRODUCTION PAGE 1 2. WEATHER DURING THE 1991 SEASON 6 3. CORRECTIONS TO PREVIOUS T.E.A. SUMMARIES 7 4. SPECIAL NOTES ON ONTARIO LEPIDOPTERA 8 4.1 The Inornate Ringlet in Middlesex & Lambton Cos. 8 4.2 The Monarch in Ontario 8 4.3 The Status of the Karner Blue & Frosted Elfin in Ontario in 1991 11 4.4 The West Virginia White in Ontario in 1991 11 4.5 Butterfly & Moth Records for Kettle Point 11 4.6 Butterflies in the Hamilton Study Area 12 4.7 Notes & Observations on the Early Hairstreak 15 4.8 A Big Day for Migrants 16 4.9 The Ocola Skipper - New to Ontario & Canada .17 4.10 The Brazilian Skipper - New to Ontario & Canada 19 4.11 Further Notes on the Zarucco Dusky Wing in Ontario 21 4.12 A Range Extension for the Large Marblewing 22 4.13 The Grayling North of Lake Superior 22 4.14 Description of an Aberrant Crescent 23 4.15 A New Foodplant for the Old World Swallowtail 24 4.16 An Owl Moth at Point Pelee 25 4.17 Butterfly Sampling in Algoma District 26 4.18 Record Early Butterfly Dates in 1991 26 4.19 Rearing Notes from Northumberland County 28 5. GENERAL SUMMARY 29 6. 1990 SUMMARY OF ONTARIO BUTTERFLIES, SKIPPERS & MOTHS 32 Hesperiidae 32 Papilionidae 42 Pieridae 44 Lycaenidae 48 Libytheidae 56 Nymphalidae 56 Apaturidae 66 Satyr1dae 66 Danaidae 70 MOTHS 72 CONTINUOUS MOTH CYCLICAL SUMMARY 85 7. -

2017, Jones Road, Near Blackhawk, RAIN (Photo: Michael Dawber)
Edited and Compiled by Rick Cavasin and Jessica E. Linton Toronto Entomologists’ Association Occasional Publication # 48-2018 European Skippers mudpuddling, July 6, 2017, Jones Road, near Blackhawk, RAIN (Photo: Michael Dawber) Dusted Skipper, April 20, 2017, Ipperwash Beach, LAMB American Snout, August 6, 2017, (Photo: Bob Yukich) Dunes Beach, PRIN (Photo: David Kaposi) ISBN: 978-0-921631-53-7 Ontario Lepidoptera 2017 Edited and Compiled by Rick Cavasin and Jessica E. Linton April 2018 Published by the Toronto Entomologists’ Association Toronto, Ontario Production by Jessica Linton TORONTO ENTOMOLOGISTS’ ASSOCIATION Board of Directors: (TEA) Antonia Guidotti: R.O.M. Representative Programs Coordinator The TEA is a non-profit educational and scientific Carolyn King: O.N. Representative organization formed to promote interest in insects, to Publicity Coordinator encourage cooperation among amateur and professional Steve LaForest: Field Trips Coordinator entomologists, to educate and inform non-entomologists about insects, entomology and related fields, to aid in the ONTARIO LEPIDOPTERA preservation of insects and their habitats and to issue Published annually by the Toronto Entomologists’ publications in support of these objectives. Association. The TEA is a registered charity (#1069095-21); all Ontario Lepidoptera 2017 donations are tax creditable. Publication date: April 2018 ISBN: 978-0-921631-53-7 Membership Information: Copyright © TEA for Authors All rights reserved. No part of this publication may be Annual dues: reproduced or used without written permission. Individual-$30 Student-free (Association finances permitting – Information on submitting records, notes and articles to beyond that, a charge of $20 will apply) Ontario Lepidoptera can be obtained by contacting: Family-$35 Jessica E. -

Anchored Phylogenomics Illuminates the Skipper Butterfly Tree of Life
Anchored phylogenomics illuminates the skipper butterfly tree of life The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Toussaint, E. F. A., J. W. Breinholt, C. Earl, A. D. Warren, A. V. Z. Brower, M. Yago, K. M. Dexter, et al. 2018. “Anchored phylogenomics illuminates the skipper butterfly tree of life.” BMC Evolutionary Biology 18 (1): 101. doi:10.1186/s12862-018-1216-z. http:// dx.doi.org/10.1186/s12862-018-1216-z. Published Version doi:10.1186/s12862-018-1216-z Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:37298562 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Toussaint et al. BMC Evolutionary Biology (2018) 18:101 https://doi.org/10.1186/s12862-018-1216-z RESEARCH ARTICLE Open Access Anchored phylogenomics illuminates the skipper butterfly tree of life Emmanuel F. A. Toussaint1* , Jesse W. Breinholt1,2, Chandra Earl1, Andrew D. Warren1, Andrew V. Z. Brower3, Masaya Yago4, Kelly M. Dexter1, Marianne Espeland5, Naomi E. Pierce6, David J. Lohman7,8,9 and Akito Y. Kawahara1 Abstract Background: Butterflies (Papilionoidea) are perhaps the most charismatic insect lineage, yet phylogenetic relationships among them remain incompletely studied and controversial. This is especially true for skippers (Hesperiidae), one of the most species-rich and poorly studied butterfly families. Methods: To infer a robust phylogenomic hypothesis for Hesperiidae, we sequenced nearly 400 loci using Anchored Hybrid Enrichment and sampled all tribes and more than 120 genera of skippers. -

Phylogeny and Evolution of Lepidoptera
EN62CH15-Mitter ARI 5 November 2016 12:1 I Review in Advance first posted online V E W E on November 16, 2016. (Changes may R S still occur before final publication online and in print.) I E N C N A D V A Phylogeny and Evolution of Lepidoptera Charles Mitter,1,∗ Donald R. Davis,2 and Michael P. Cummings3 1Department of Entomology, University of Maryland, College Park, Maryland 20742; email: [email protected] 2Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560 3Laboratory of Molecular Evolution, Center for Bioinformatics and Computational Biology, University of Maryland, College Park, Maryland 20742 Annu. Rev. Entomol. 2017. 62:265–83 Keywords Annu. Rev. Entomol. 2017.62. Downloaded from www.annualreviews.org The Annual Review of Entomology is online at Hexapoda, insect, systematics, classification, butterfly, moth, molecular ento.annualreviews.org systematics This article’s doi: Access provided by University of Maryland - College Park on 11/20/16. For personal use only. 10.1146/annurev-ento-031616-035125 Abstract Copyright c 2017 by Annual Reviews. Until recently, deep-level phylogeny in Lepidoptera, the largest single ra- All rights reserved diation of plant-feeding insects, was very poorly understood. Over the past ∗ Corresponding author two decades, building on a preceding era of morphological cladistic stud- ies, molecular data have yielded robust initial estimates of relationships both within and among the ∼43 superfamilies, with unsolved problems now yield- ing to much larger data sets from high-throughput sequencing. Here we summarize progress on lepidopteran phylogeny since 1975, emphasizing the superfamily level, and discuss some resulting advances in our understanding of lepidopteran evolution. -

Influence of Habitat and Bat Activity on Moth Community Composition and Seasonal Phenology Across Habitat Types
INFLUENCE OF HABITAT AND BAT ACTIVITY ON MOTH COMMUNITY COMPOSITION AND SEASONAL PHENOLOGY ACROSS HABITAT TYPES BY MATTHEW SAFFORD THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology in the Graduate College of the University of Illinois at Urbana-Champaign, 2018 Urbana, Illinois Advisor: Assistant Professor Alexandra Harmon-Threatt, Chair and Director of Research ABSTRACT Understanding the factors that influence moth diversity and abundance is important for monitoring moth biodiversity and developing conservation strategies. Studies of moth habitat use have primarily focused on access to host plants used by specific moth species. How vegetation structure influences moth communities within and between habitats and mediates the activity of insectivorous bats is understudied. Previous research into the impact of bat activity on moths has primarily focused on interactions in a single habitat type or a single moth species of interest, leaving a large knowledge gap on how habitat structure and bat activity influence the composition of moth communities across habitat types. I conducted monthly surveys at sites in two habitat types, restoration prairie and forest. Moths were collected using black light bucket traps and identified to species. Bat echolocation calls were recorded using ultrasonic detectors and classified into phonic groups to understand how moth community responds to the presence of these predators. Plant diversity and habitat structure variables, including tree diameter at breast height, ground cover, and vegetation height were measured during summer surveys to document how differences in habitat structure between and within habitats influences moth diversity. I found that moth communities vary significantly between habitat types. -

Phylogenomics Reveals Major Diversification Rate Shifts in The
bioRxiv preprint doi: https://doi.org/10.1101/517995; this version posted January 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Phylogenomics reveals major diversification rate shifts in the evolution of silk moths and 2 relatives 3 4 Hamilton CA1,2*, St Laurent RA1, Dexter, K1, Kitching IJ3, Breinholt JW1,4, Zwick A5, Timmermans 5 MJTN6, Barber JR7, Kawahara AY1* 6 7 Institutional Affiliations: 8 1Florida Museum of Natural History, University of Florida, Gainesville, FL 32611 USA 9 2Department of Entomology, Plant Pathology, & Nematology, University of Idaho, Moscow, ID 10 83844 USA 11 3Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK 12 4RAPiD Genomics, 747 SW 2nd Avenue #314, Gainesville, FL 32601. USA 13 5Australian National Insect Collection, CSIRO, Clunies Ross St, Acton, ACT 2601, Canberra, 14 Australia 15 6Department of Natural Sciences, Middlesex University, The Burroughs, London NW4 4BT, UK 16 7Department of Biological Sciences, Boise State University, Boise, ID 83725, USA 17 *Correspondence: [email protected] (CAH) or [email protected] (AYK) 18 19 20 Abstract 21 The silkmoths and their relatives (Bombycoidea) are an ecologically and taxonomically 22 diverse superfamily that includes some of the most charismatic species of all the Lepidoptera. 23 Despite displaying some of the most spectacular forms and ecological traits among insects, 24 relatively little attention has been given to understanding their evolution and the drivers of 25 their diversity. -

Butterfly Blues Pdf, Epub, Ebook
BUTTERFLY BLUES PDF, EPUB, EBOOK Carolyn Keene,Mr Peter Francis | 96 pages | 01 Apr 2015 | SIMON & SCHUSTER | 9781481414708 | English | New York, United States Butterfly Blues PDF Book Australian Museum. You must be a registered user to use the IMDb rating plugin. Protect from winter wet. C W marked it as to-read Dec 16, My Region USA. Bloom Time: April to frost. Instead, when the wings are open you can see brilliant iridescent blue bordered by black. Recorded food plants are Lathyrus species, Vicia species, Vicia cracca , Oxytropis campestris , bird's foot trefoil Lotus corniculatus , Oxytropis pyrenaica , Astragalus aristatus , Astragalus onobrychis , Astragalus pinetorum , black medick Medicago lupulina , Medicago romanica , Medicago falcata , common restharrow Ononis repens , wild thyme Thymus serpyllum , lesser trefoil Trifolium dubium , Trifolium pratense and white clover Trifolium repens. Join now and start creating your dream garden! The Atala butterfly Eumaeus atala Poey is a rare butterfly with a limited distribution in South Florida. Namespaces Article Talk. Gardening Help Search. These butterflies are large, with a wing span of 3 to 3. Runtime: 3 min. Want to Read Currently Reading Read. Dwarfing habit and lengthy bloom period make these excellent plants for grouping or massing in border fronts and rock gardens. During the first instar, larva emerge and eat away the crown of the egg. Jeanette marked it as to-read May 24, Where are you based? Remove spent flowers to encourage additional bloom. We use cookies on this website, you can read about them here. Help Learn to edit Community portal Recent changes Upload file. All rights reserved. -
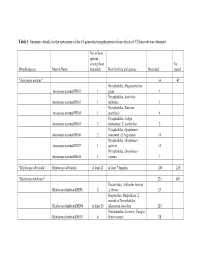
Table 1: Summary Details for the Specimens of the 16 Generalist Morphospecies from Which a COI Barcode Was Obtained
Table 1: Summary details for the specimens of the 16 generalist morphospecies from which a COI barcode was obtained No. of host species among those No. Morphospecies Interim Name barcoded Host families and species Barcoded reared "Anoxynops auratus" 34 49 Nymphalidae, Magneuptychia Anoxynops auratusDHJ01 1 gigas 1 Nymphalidae, Antirrhea Anoxynops auratusDHJ03 1 miltiades 1 Nymphalidae, Siderone Anoxynops auratusDHJ04 1 marthesia 4 Nymphalidae, Caligo Anoxynops auratusDHJ05 2 telamonius, C. eurilochus 2 Nymphalidae, Opsiphanes Anoxynops auratusDHJ06 2 tamarindi, O. bogotanus 10 Nymphalidae, Opsiphanes Anoxynops auratusDHJ07 1 quiteria 10 Nymphalidae, Opsiphanes Anoxynops auratusDHJ08 1 cassina 7 "Blepharipa albicauda" Blepharipa albicauda at least 21 at least 7 families 108 226 "Blepharipa fimbriata" 221 483 Hesperiidae, Achlyodes busirus, Blepharipa fimbriataDHJ01 2 A. thraso 23 Hesperiidae, Hesperiinae; 2 records of Nymphalidae, Blepharipa fimbriataDHJ04 at least 24 Manataria maculata 123 Notodontidae, Lirimiris, Farigia, Blepharipa fimbriataDHJ11 4 Heterocampa 28 Saturniidae, Syssphinx, Blepharipa fimbriataDHJ12 4 Ptiloscola dargei 47 "Chetogena scutellaris" 82 172 Chetogena scutellarisDHJ01 at least 22 at least 12 families 38 Chetogena scutellarisDHJ02 at least 20 at least 9 families 44 "Drino piceiventris" 223 455 Sphingidae, Callionima Drino piceiventrisDHJ03 1 denticulata 22 Drino piceiventrisDHJ04 1 Sphingidae, Adhemarius ypsilon 13 Sphingidae, Manduca, Erinnyis, Drino piceiventrisDHJ05 12 Eumorpha, Madoryx; rain forest 46 Sphingidae, Manduca, Protambulyx, Madoryx, dry Drino piceiventrisDHJ06 6 forest 16 Sphingidae, Enyo, Eumorpha, Aleuron, on Drino piceiventrisDHJ07 5 Dilleniaceae/Vitaceae 27 Sphingidae, Cautethia spuria, C. Drino piceiventrisDHJ08 2 yucatana 4 Sphingidae, Erinnyis, Drino piceiventrisDHJ09 3 Eupyrrhoglossum 4 Sphingidae, Enyo, Aleuron, Drino piceiventrisDHJ10 5 Erinnyis 14 Drino piceiventrisDHJ11 3 Sphinigdae, Pachylia, Erinnyis 24 Drino piceiventrisDHJ13 2 Sphingidae, Enyo 5 Sphingidae, Aellopos fadus, A. -

Impacts of Native and Non-Native Plants on Urban Insect Communities: Are Native Plants Better Than Non-Natives?
Impacts of Native and Non-native plants on Urban Insect Communities: Are Native Plants Better than Non-natives? by Carl Scott Clem A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Master of Science Auburn, Alabama December 12, 2015 Key Words: native plants, non-native plants, caterpillars, natural enemies, associational interactions, congeneric plants Copyright 2015 by Carl Scott Clem Approved by David Held, Chair, Associate Professor: Department of Entomology and Plant Pathology Charles Ray, Research Fellow: Department of Entomology and Plant Pathology Debbie Folkerts, Assistant Professor: Department of Biological Sciences Robert Boyd, Professor: Department of Biological Sciences Abstract With continued suburban expansion in the southeastern United States, it is increasingly important to understand urbanization and its impacts on sustainability and natural ecosystems. Expansion of suburbia is often coupled with replacement of native plants by alien ornamental plants such as crepe myrtle, Bradford pear, and Japanese maple. Two projects were conducted for this thesis. The purpose of the first project (Chapter 2) was to conduct an analysis of existing larval Lepidoptera and Symphyta hostplant records in the southeastern United States, comparing their species richness on common native and alien woody plants. We found that, in most cases, native plants support more species of eruciform larvae compared to aliens. Alien congener plant species (those in the same genus as native species) supported more species of larvae than alien, non-congeners. Most of the larvae that feed on alien plants are generalist species. However, most of the specialist species feeding on alien plants use congeners of native plants, providing evidence of a spillover, or false spillover, effect. -

Mimallonidae, Cicinninae, Psychocampini) and the Description of the Female of P
ST LAURENT: Three new Psychocampa TROP. LEPID. RES., 29(1): 1-11, 2019 1 Three new species of Psychocampa Grote and Robinson (Mimallonidae, Cicinninae, Psychocampini) and the description of the female of P. kohlli Herbin Ryan A. St Laurent1,2 1Department of Biology, University of Florida, Gainesville, FL 32611, USA; 2McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, 3215 Hull Road, Gainesville, FL 32611-2710 USA; [email protected] Date of issue online: 3 May 2019 Zoobank Registered: urn:lsid:zoobank.org:pub:B0F0E209-60A6-419B-A8D0-E60EDCAA5CC0 Electronic copies (ISSN 2575-9256) in PDF format at: http://journals.fcla.edu/troplep; https://zenodo.org; archived by the Institutional Repository at the University of Florida (IR@UF), http://ufdc.ufl.edu/ufir;DOI : 10.5281/zenodo.2647560 © The author(s). This is an open access article distributed under the Creative Commons license CC BY-NC 4.0 (https://creativecommons.org/ licenses/by-nc/4.0/). Abstract: Three new species of Psychocampa Grote and Robinson, 1867 are described from Central and South America: one from Costa Rica and Panama: P. ursa n. sp.; and two from Peru: P. antonkozlovi n. sp. and P. yuliyakovalevae n. sp.. The female of the somewhat rarely reported P. kohlli Herbin, 2012 is figured and described, along with its genitalia, for the first time. Four new country records are also provided for P. kohlli. Keywords: Andes Mountains, Costa Rica, Mimallonoidea, Neotropical, Panama, Peru INTRODUCTION to Psychocampa from Cicinnus, and several others from Psychocampa to Cicinnus and to other genera, illustrating Recent phylogenetic studies have started to define the that many species had historically been incorrectly placed various clades of Mimallonidae (St Laurent et al., 2018). -

Mimallonidae (Lepidoptera)
www.biotaxa.org/rce. ISSN 0718-8994 (online) Revista Chilena de Entomología (2021) 47 (3): 501-511. Research Article Hidden treasures: Mimallonidae (Lepidoptera) from the Museo Javeriano de Historia Natural, with descriptions of the female of Bedosiallo moengus (Schaus, 1928), and a new species of Gonogramma Boisduval, 1872 Tesoros ocultos: Mimallonidae (Lepidoptera) del Museo Javeriano de Historia Natural, descripción de la hembra de Bedosiallo moengus (Schaus, 1928), y una nueva especie de Gonogramma Boisduval, 1872 Liliana Prada-Lara1 and Ryan A. St Laurent2* 1Laboratory of Entomology. Pontificia Universidad Javeriana, Bogotá, Colombia. E-mail: lilianapradalara@gmail. com. 2McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, Gainesville, FL 32611, USA. *[email protected] ZooBank: urn:lsid:zoobank.org:pub:41DB45D6-01DC-4299-AC28-CFB498DF0A8B https://doi.org/10.35249/rche.47.3.21.08 Abstract. We present the first list focused on Mimallonidae from a Colombian biological collection, the Museo Javeriano de Historia Natural “Lorenzo Uribe, S.J.” (MPUJ) of the Pontificia Universidad Javeriana in Bogotá. We report nine species and seven genera, and we highlight the museum’s hidden treasures: the first known female ofBedosiallo moengus (Schaus, 1928) and a new species, Gonogramma faguai nov. sp. Key words: Colombia; MPUJ; Neotropical Region; Sack-bearer moths. Resumen. Se presenta el primer listado de la familia Mimallonidae para una colección biológica colombiana, el Museo Javeriano de Historia Natural “Lorenzo Uribe, S.J.” (MPUJ) de la Pontificia Universidad Javeriana en Bogotá. Se reportan nueve especies y siete géneros de esta familia de polillas, donde se destacan los tesoros ocultos del museo: la primera hembra conocida de Bedosiallo moengus (Schaus, 1928) y una nueva especie, Gonogramma faguai sp. -

A Survey of Macrolepidopterous Moths
Banisteria, Number 17, 2001 © 2001 by the Virginia Natural History Society An Update to the Survey of Macrolepidopteran Moths Near Vontay, Hanover County, Virginia J. Christopher Ludwig Virginia Department of Conservation and Recreation Division of Natural Heritage 217 Governor Street Richmond, Virginia 23219 INTRODUCTION 1992) and Kentucky (Covell, 1999) with 708 and 616 species, respectively, an estimate of the percentage of This paper updates the list of macrolepidopteran the Commonwealth’s noctuid fauna represented at the moth (= “macro moth” or “macro”) species collected at Vontay site is about 43 to 49%. a site on the Virginia Piedmont, 2 km W of Vontay in An updated species-accumulation curve (Fig. 1) for western Hanover County. The study site, methodology, this study indicates that additional macros will be and original species list are given in Ludwig (2000). recorded at the site should collections continue. The list is updated with new collections as well as Depending upon the projected trajectory of the curve, identifications of additional specimens collected during estimates of the total number of taxa recorded from this the original study period. The new collections extend site over 10 years range from 560 to 660 macro-moth the study period from two years (30 October 1996 to 28 species, representing perhaps as much as 50% of the October 1998) to nearly four years (30 October 1996 to state’s estimated total macro fauna. 14 September 2000) and increase the number of collection nights from 311 to >400. The species list from this study, along with others being gathered Table 1. Summary of macro-moths encountered during throughout Virginia by the Virginia Department of this study by family given in order of Hodges et al.