Impacts of Native and Non-Native Plants on Urban Insect Communities: Are Native Plants Better Than Non-Natives?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
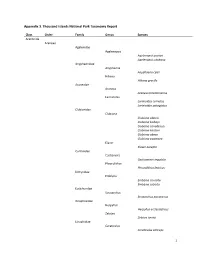
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Type Material of a Pine Web-Spinning Sawfly, Acantholyda Sasakii (Yano
Bull. Natl. Mus. Nat. Sci., Ser. A, 39(3), pp. 131–132, August 22, 2013 Type Material of a Pine Web-spinning Sawfly, Acantholyda sasakii (Yano, 1916) (Hymenoptera, Pamphiliidae) Akihiko Shinohara Department of Zoology, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki, 305–0005 Japan E-mail: [email protected] (Received 1 June 2013; accepted 12 July 2013) Abstract The type material of Acantholyda sasakii (Yano, 1916), once thought to be lost, has been found in the collection of the University Museum, the University of Tokyo, Tokyo. A lectotype is designated for the taxon. An examination of the lectotype has shown that the current interpretation of the taxon is correct. Key words : Hymenoptera, Pamphiliidae, Acantholyda sasakii, lectotype designation. Acantholyda sasakii (Yano, 1916) is a conifer- 1918] from Europe and named it “Lgda [sic] feeding, web-spinning sawfly occurring in Hon- sasakii”. Takeuchi (1930) transferred it to the shu, Japan (Shinohara, 1995, 2001). Sasaki genus Acantholyda Costa, 1894. Although Sasa- (1901) first described this species as “Tenthredo ki’s original material has never been studied, as it pratensis, F. var.?” without giving the number of was thought to be lost (Shinohara, 1995), the specimens he examined. Yano (1916) pointed out species is quite characteristic and easily recog- that Sasaki’s species differed from “Tenthredo nized by the features given by Sasaki (1901). pratensis F.” [=Tenthredo stellata Christ, =Acan- Chûjirô Sasaki (1857–1938) was a professor tholyda (Itycorsia) posticalis pinivora Enslin, of Entomology at the College of Agriculture, Fig. 1. Lectotype of Lyda sasakii Yano, 1916. 132 Akihiko Shinohara Tokyo Imperial University (currently the Univer- of the antennae and the right fore tibia and tarsus sity of Tokyo), and his insect collection is sup- are missing, and the left wings are detached from posed to have been deposited in the college. -

Insect Survey of Four Longleaf Pine Preserves
A SURVEY OF THE MOTHS, BUTTERFLIES, AND GRASSHOPPERS OF FOUR NATURE CONSERVANCY PRESERVES IN SOUTHEASTERN NORTH CAROLINA Stephen P. Hall and Dale F. Schweitzer November 15, 1993 ABSTRACT Moths, butterflies, and grasshoppers were surveyed within four longleaf pine preserves owned by the North Carolina Nature Conservancy during the growing season of 1991 and 1992. Over 7,000 specimens (either collected or seen in the field) were identified, representing 512 different species and 28 families. Forty-one of these we consider to be distinctive of the two fire- maintained communities principally under investigation, the longleaf pine savannas and flatwoods. An additional 14 species we consider distinctive of the pocosins that occur in close association with the savannas and flatwoods. Twenty nine species appear to be rare enough to be included on the list of elements monitored by the North Carolina Natural Heritage Program (eight others in this category have been reported from one of these sites, the Green Swamp, but were not observed in this study). Two of the moths collected, Spartiniphaga carterae and Agrotis buchholzi, are currently candidates for federal listing as Threatened or Endangered species. Another species, Hemipachnobia s. subporphyrea, appears to be endemic to North Carolina and should also be considered for federal candidate status. With few exceptions, even the species that seem to be most closely associated with savannas and flatwoods show few direct defenses against fire, the primary force responsible for maintaining these communities. Instead, the majority of these insects probably survive within this region due to their ability to rapidly re-colonize recently burned areas from small, well-dispersed refugia. -

Use of a Native and an Exotic Malvaceae by the Little Known Skipper Pyrgus Bocchoris Trisignatus (Mabille) (Hesperiidae) in Northern Chile
VOLUME 67, N UMBER 3 GENERAL NOTES 225 Journal of the Lepidopterists’ Society 67(3), 2013, 225-226 USE OF A NATIVE AND AN EXOTIC MALVACEAE BY THE LITTLE KNOWN SKIPPER PYRGUS BOCCHORIS TRISIGNATUS (MABILLE) (HESPERIIDAE) IN NORTHERN CHILE Additional key words: Folivorous, Naturalized, Malva nicaeensis, Tarasa operculata Many butterflies are highly specialized in their use of characterized by a typical fauna and flora (Luebert & host plants. Some are monophagous (Brückmann et al. Pliscoff 2006). This skipper is one of the more frequently 2011); at least at a local scale (Jordano et al. 1990, Vargas observed butterflies in many of these situations, 2012). Despite this tendency towards specialization, including relatively pristine areas and also highly however, oviposition by native butterflies on exotic modified agricultural lands. Shapiro (1991) indicated that plants, and the subsequent successful larval a Chilean representative of P. bocchoris (i.e.: trisignatus ) development, has been documented many times within is associated with weedy mallows (Malvaceae), but the New World fauna and is probably a global nothing more was published thereafter dealing with the phenomenon (Shapiro 2006). These host range shifts field biology of this skipper. Thus, the objective of this have been remarkably well studied in California, USA, paper is to document two Malvaceae host plants for P. b. where alien hosts are very important for the maintenance trisignatus based on field collections performed in of the native butterfly fauna in both urban and suburban northern Chile. environments (Shapiro 2002, Graves & Shapiro 2003). In October 2008, some Hesperiidae larvae were Recently, Jahner et al. (2011) have shown that the use of collected on leaves of the exotic mallow Malva nicaeensis exotic hosts is predicted by geographic range and native All. -

Maine State Legislature
MAINE STATE LEGISLATURE The following document is provided by the LAW AND LEGISLATIVE DIGITAL LIBRARY at the Maine State Law and Legislative Reference Library http://legislature.maine.gov/lawlib Reproduced from scanned originals with text recognition applied (searchable text may contain some errors and/or omissions) FOREST & SHADE TREE INSECT & DISEASE CONDITIONS FOR MAINE A Surrmary of the 1988 Situation Insect & Disease Management Division Maine Forest Service Surrmary Report No. 3 MAINE DEPARTMENT OF CONSERVATION March 1989 Augusta, Maine C O N T E N T S Page Introduction 1 Highlights of Division Activities for 1988 .............................. 1 Organizational Chart (I & DM) ••••••••• 2 Entomology Technician Districts (Map) ••• 3 Publications ....................................... 4 1988 Pest Summary 5 (A) Forest Pests - Softwoods 6 Insects ............... ~ .......... ~ ................................ 6 Diseases 10 (B) Forest Pests Hardwoods 12 Insects ......................................................... 12 Diseases ........................................................... 19 (C) Plantation, Regeneration, Nursery and Christmas Tree Pests (Conifers Only) ............................................................... 21 Insects .............................................. • ............. 21 Diseases and Miscellaneous Problems ....... •........................ 25 (D) Shade Tree, Ornamental and Miscellaneous Pests 26 Insects and Ticks .................................................. 26 Diseases and Miscellaneous Problems -

A New Leaf-Mining Moth from New Zealand, Sabulopteryx Botanica Sp
A peer-reviewed open-access journal ZooKeys 865: 39–65A new (2019) leaf-mining moth from New Zealand, Sabulopteryx botanica sp. nov. 39 doi: 10.3897/zookeys.865.34265 MONOGRAPH http://zookeys.pensoft.net Launched to accelerate biodiversity research A new leaf-mining moth from New Zealand, Sabulopteryx botanica sp. nov. (Lepidoptera, Gracillariidae, Gracillariinae), feeding on the rare endemic shrub Teucrium parvifolium (Lamiaceae), with a revised checklist of New Zealand Gracillariidae Robert J.B. Hoare1, Brian H. Patrick2, Thomas R. Buckley1,3 1 New Zealand Arthropod Collection (NZAC), Manaaki Whenua–Landcare Research, Private Bag 92170, Auc- kland, New Zealand 2 Wildlands Consultants Ltd, PO Box 9276, Tower Junction, Christchurch 8149, New Ze- aland 3 School of Biological Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand Corresponding author: Robert J.B. Hoare ([email protected]) Academic editor: E. van Nieukerken | Received 4 March 2019 | Accepted 3 May 2019 | Published 22 Jul 2019 http://zoobank.org/C1E51F7F-B5DF-4808-9C80-73A10D5746CD Citation: Hoare RJB, Patrick BH, Buckley TR (2019) A new leaf-mining moth from New Zealand, Sabulopteryx botanica sp. nov. (Lepidoptera, Gracillariidae, Gracillariinae), feeding on the rare endemic shrub Teucrium parvifolium (Lamiaceae), with a revised checklist of New Zealand Gracillariidae. ZooKeys 965: 39–65. https://doi.org/10.3897/ zookeys.865.34265 Abstract Sabulopteryx botanica Hoare & Patrick, sp. nov. (Lepidoptera, Gracillariidae, Gracillariinae) is described as a new species from New Zealand. It is regarded as endemic, and represents the first record of its genus from the southern hemisphere. Though diverging in some morphological features from previously de- scribed species, it is placed in genus Sabulopteryx Triberti, based on wing venation, abdominal characters, male and female genitalia and hostplant choice; this placement is supported by phylogenetic analysis based on the COI mitochondrial gene. -

Errata and First Update to the 2010 Checklist of the Lepidoptera Of
Errata and first uppppdate to the 2010 checklist of the Lepidoptera of Alberta Gregory R. Pohl, Jason J Dombroskie, Jean‐François Landry, Charles D Bird, and Vazrick Nazari lead author contact: [email protected] Introduction: Since the Annotated list of the Lepidoptera of Alberta was published in March 2010 (Pohl et al. 2010), a few typographical and nomenclatural errors have come to the authors' attention, as well as three erroneous AB records that were inadvertently omitted from that publication. Additionally, a considerable number of new AB species records have been brought to our attention since that checklist went to press. As expected, most are microlepidoptera. We detail all these items below, in what we hope will be a regular series of addenda to the AB list. If you are aware of further errors or additions to the AB Lepidoptera list, please contact the authors. Wit hin the NidNoctuoidea, there are a few minor iiiinconsistencies in the order of species wihiithin genera, and in the order of genera within tribes or subtribes, as compared to the sequence published by Lafontaine & Schmidt (2010). As well, the sequence of tribes in the AB list does not exactly match that of Lafontaine & Schmidt (2010), particularly in the Erebinae. We are not detailing those minor differences here unless they involve a move to a new genus or new higher taxonomic category. Errata: Abstract, p. 2, line 10, should read "1530... annotations are given" 41 Nemapogon granella (p. 55). Add Kearfott (1905) to the AB literature records. 78 Caloptilia syringella (p. 60). This species should be placed in the genus Gracillaria as per De Prins & De Prins (2005). -

Moths of North Carolina - Early Draft 1
Noctuidae Achatia distincta Distinct Quaker Moth 20 n=12 • • High Mt. • • • • N 10 • •• u • • • m • • • • b • 0 • • e • • • r 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 • 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 NC counties: 27 • Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec • o 20 • f n=39 • = Sighting or Collection Low Mt. High counts of: in NC since 2001 F • = Not seen since 2001 l 10 30 - Ashe - 2000-05-02 • i 8 - Macon - 2001-04-21 g Status Rank h 6 - Ashe - 2000-05-02 0 NC US NC Global t 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 D Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec a 20 20 t n=35 n=7 e Pd CP s 10 10 0 0 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Three periods to each month: 1-10 / 11-20 / 21-31 FAMILY: Noctuidae SUBFAMILY: Noctuinae TRIBE: Orthosiini TAXONOMIC_COMMENTS: A monotypic genus found across most of eastern North America and throughout North Carolina. -

2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory
2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory ........................................................................................... 3 Alaska ... ........................................ ............................................................... 3 LEPIDOPTERISTS Zone 2: British Columbia .................................................... ........................ ............ 6 Idaho .. ... ....................................... ................................................................ 6 Oregon ........ ... .... ........................ .. .. ............................................................ 10 SOCIETY Volume 53 Supplement Sl Washington ................................................................................................ 14 Zone 3: Arizona ............................................................ .................................... ...... 19 The Lepidopterists' Society is a non-profo California ............... ................................................. .............. .. ................... 2 2 educational and scientific organization. The Nevada ..................................................................... ................................ 28 object of the Society, which was formed in Zone 4: Colorado ................................ ... ............... ... ...... ......................................... 2 9 May 1947 and formally constituted in De Montana .................................................................................................... 51 cember -

Butterflies and Moths of Centre County, Pennsylvania, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

An Annotated List of the Lepidoptera of Alberta, Canada
A peer-reviewed open-access journal ZooKeys 38: 1–549 (2010) Annotated list of the Lepidoptera of Alberta, Canada 1 doi: 10.3897/zookeys.38.383 MONOGRAPH www.pensoftonline.net/zookeys Launched to accelerate biodiversity research An annotated list of the Lepidoptera of Alberta, Canada Gregory R. Pohl1, Gary G. Anweiler2, B. Christian Schmidt3, Norbert G. Kondla4 1 Editor-in-chief, co-author of introduction, and author of micromoths portions. Natural Resources Canada, Northern Forestry Centre, 5320 - 122 St., Edmonton, Alberta, Canada T6H 3S5 2 Co-author of macromoths portions. University of Alberta, E.H. Strickland Entomological Museum, Department of Biological Sciences, Edmonton, Alberta, Canada T6G 2E3 3 Co-author of introduction and macromoths portions. Canadian Food Inspection Agency, Canadian National Collection of Insects, Arachnids and Nematodes, K.W. Neatby Bldg., 960 Carling Ave., Ottawa, Ontario, Canada K1A 0C6 4 Author of butterfl ies portions. 242-6220 – 17 Ave. SE, Calgary, Alberta, Canada T2A 0W6 Corresponding authors: Gregory R. Pohl ([email protected]), Gary G. Anweiler ([email protected]), B. Christian Schmidt ([email protected]), Norbert G. Kondla ([email protected]) Academic editor: Donald Lafontaine | Received 11 January 2010 | Accepted 7 February 2010 | Published 5 March 2010 Citation: Pohl GR, Anweiler GG, Schmidt BC, Kondla NG (2010) An annotated list of the Lepidoptera of Alberta, Canada. ZooKeys 38: 1–549. doi: 10.3897/zookeys.38.383 Abstract Th is checklist documents the 2367 Lepidoptera species reported to occur in the province of Alberta, Can- ada, based on examination of the major public insect collections in Alberta and the Canadian National Collection of Insects, Arachnids and Nematodes.