Butterfly Blues Pdf, Epub, Ebook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA Barcodes Reveal Deeply Neglected Diversity and Numerous Invasions of Micromoths in Madagascar
Genome DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in Madagascar Journal: Genome Manuscript ID gen-2018-0065.R2 Manuscript Type: Article Date Submitted by the 17-Jul-2018 Author: Complete List of Authors: Lopez-Vaamonde, Carlos; Institut National de la Recherche Agronomique (INRA), ; Institut de Recherche sur la Biologie de l’Insecte (IRBI), Sire, Lucas; Institut de Recherche sur la Biologie de l’Insecte Rasmussen,Draft Bruno; Institut de Recherche sur la Biologie de l’Insecte Rougerie, Rodolphe; Institut Systématique, Evolution, Biodiversité (ISYEB), Wieser, Christian; Landesmuseum für Kärnten Ahamadi, Allaoui; University of Antananarivo, Department Entomology Minet, Joël; Institut de Systematique Evolution Biodiversite deWaard, Jeremy; Biodiversity Institute of Ontario, University of Guelph, Decaëns, Thibaud; Centre d'Ecologie Fonctionnelle et Evolutive (CEFE UMR 5175, CNRS–Université de Montpellier–Université Paul-Valéry Montpellier–EPHE), , CEFE UMR 5175 CNRS Lees, David; Natural History Museum London Keyword: Africa, invasive alien species, Lepidoptera, Malaise trap, plant pests Is the invited manuscript for consideration in a Special 7th International Barcode of Life Issue? : https://mc06.manuscriptcentral.com/genome-pubs Page 1 of 57 Genome 1 DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in 2 Madagascar 3 4 5 Carlos Lopez-Vaamonde1,2, Lucas Sire2, Bruno Rasmussen2, Rodolphe Rougerie3, 6 Christian Wieser4, Allaoui Ahamadi Allaoui 5, Joël Minet3, Jeremy R. deWaard6, Thibaud 7 Decaëns7, David C. Lees8 8 9 1 INRA, UR633, Zoologie Forestière, F- 45075 Orléans, France. 10 2 Institut de Recherche sur la Biologie de l’Insecte, UMR 7261 CNRS Université de Tours, UFR 11 Sciences et Techniques, Tours, France. -

Butterflies of Ontario & Summaries of Lepidoptera
ISBN #: 0-921631-12-X BUTTERFLIES OF ONTARIO & SUMMARIES OF LEPIDOPTERA ENCOUNTERED IN ONTARIO IN 1991 BY A.J. HANKS &Q.F. HESS PRODUCTION BY ALAN J. HANKS APRIL 1992 CONTENTS 1. INTRODUCTION PAGE 1 2. WEATHER DURING THE 1991 SEASON 6 3. CORRECTIONS TO PREVIOUS T.E.A. SUMMARIES 7 4. SPECIAL NOTES ON ONTARIO LEPIDOPTERA 8 4.1 The Inornate Ringlet in Middlesex & Lambton Cos. 8 4.2 The Monarch in Ontario 8 4.3 The Status of the Karner Blue & Frosted Elfin in Ontario in 1991 11 4.4 The West Virginia White in Ontario in 1991 11 4.5 Butterfly & Moth Records for Kettle Point 11 4.6 Butterflies in the Hamilton Study Area 12 4.7 Notes & Observations on the Early Hairstreak 15 4.8 A Big Day for Migrants 16 4.9 The Ocola Skipper - New to Ontario & Canada .17 4.10 The Brazilian Skipper - New to Ontario & Canada 19 4.11 Further Notes on the Zarucco Dusky Wing in Ontario 21 4.12 A Range Extension for the Large Marblewing 22 4.13 The Grayling North of Lake Superior 22 4.14 Description of an Aberrant Crescent 23 4.15 A New Foodplant for the Old World Swallowtail 24 4.16 An Owl Moth at Point Pelee 25 4.17 Butterfly Sampling in Algoma District 26 4.18 Record Early Butterfly Dates in 1991 26 4.19 Rearing Notes from Northumberland County 28 5. GENERAL SUMMARY 29 6. 1990 SUMMARY OF ONTARIO BUTTERFLIES, SKIPPERS & MOTHS 32 Hesperiidae 32 Papilionidae 42 Pieridae 44 Lycaenidae 48 Libytheidae 56 Nymphalidae 56 Apaturidae 66 Satyr1dae 66 Danaidae 70 MOTHS 72 CONTINUOUS MOTH CYCLICAL SUMMARY 85 7. -

BÖCEKLERİN SINIFLANDIRILMASI (Takım Düzeyinde)
BÖCEKLERİN SINIFLANDIRILMASI (TAKIM DÜZEYİNDE) GÖKHAN AYDIN 2016 Editör : Gökhan AYDIN Dizgi : Ziya ÖNCÜ ISBN : 978-605-87432-3-6 Böceklerin Sınıflandırılması isimli eğitim amaçlı hazırlanan bilgisayar programı için lütfen aşağıda verilen linki tıklayarak programı ücretsiz olarak bilgisayarınıza yükleyin. http://atabeymyo.sdu.edu.tr/assets/uploads/sites/76/files/siniflama-05102016.exe Eğitim Amaçlı Bilgisayar Programı ISBN: 978-605-87432-2-9 İçindekiler İçindekiler i Önsöz vi 1. Protura - Coneheads 1 1.1 Özellikleri 1 1.2 Ekonomik Önemi 2 1.3 Bunları Biliyor musunuz? 2 2. Collembola - Springtails 3 2.1 Özellikleri 3 2.2 Ekonomik Önemi 4 2.3 Bunları Biliyor musunuz? 4 3. Thysanura - Silverfish 6 3.1 Özellikleri 6 3.2 Ekonomik Önemi 7 3.3 Bunları Biliyor musunuz? 7 4. Microcoryphia - Bristletails 8 4.1 Özellikleri 8 4.2 Ekonomik Önemi 9 5. Diplura 10 5.1 Özellikleri 10 5.2 Ekonomik Önemi 10 5.3 Bunları Biliyor musunuz? 11 6. Plocoptera – Stoneflies 12 6.1 Özellikleri 12 6.2 Ekonomik Önemi 12 6.3 Bunları Biliyor musunuz? 13 7. Embioptera - webspinners 14 7.1 Özellikleri 15 7.2 Ekonomik Önemi 15 7.3 Bunları Biliyor musunuz? 15 8. Orthoptera–Grasshoppers, Crickets 16 8.1 Özellikleri 16 8.2 Ekonomik Önemi 16 8.3 Bunları Biliyor musunuz? 17 i 9. Phasmida - Walkingsticks 20 9.1 Özellikleri 20 9.2 Ekonomik Önemi 21 9.3 Bunları Biliyor musunuz? 21 10. Dermaptera - Earwigs 23 10.1 Özellikleri 23 10.2 Ekonomik Önemi 24 10.3 Bunları Biliyor musunuz? 24 11. Zoraptera 25 11.1 Özellikleri 25 11.2 Ekonomik Önemi 25 11.3 Bunları Biliyor musunuz? 26 12. -

Anchored Phylogenomics Illuminates the Skipper Butterfly Tree of Life
Anchored phylogenomics illuminates the skipper butterfly tree of life The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Toussaint, E. F. A., J. W. Breinholt, C. Earl, A. D. Warren, A. V. Z. Brower, M. Yago, K. M. Dexter, et al. 2018. “Anchored phylogenomics illuminates the skipper butterfly tree of life.” BMC Evolutionary Biology 18 (1): 101. doi:10.1186/s12862-018-1216-z. http:// dx.doi.org/10.1186/s12862-018-1216-z. Published Version doi:10.1186/s12862-018-1216-z Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:37298562 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Toussaint et al. BMC Evolutionary Biology (2018) 18:101 https://doi.org/10.1186/s12862-018-1216-z RESEARCH ARTICLE Open Access Anchored phylogenomics illuminates the skipper butterfly tree of life Emmanuel F. A. Toussaint1* , Jesse W. Breinholt1,2, Chandra Earl1, Andrew D. Warren1, Andrew V. Z. Brower3, Masaya Yago4, Kelly M. Dexter1, Marianne Espeland5, Naomi E. Pierce6, David J. Lohman7,8,9 and Akito Y. Kawahara1 Abstract Background: Butterflies (Papilionoidea) are perhaps the most charismatic insect lineage, yet phylogenetic relationships among them remain incompletely studied and controversial. This is especially true for skippers (Hesperiidae), one of the most species-rich and poorly studied butterfly families. Methods: To infer a robust phylogenomic hypothesis for Hesperiidae, we sequenced nearly 400 loci using Anchored Hybrid Enrichment and sampled all tribes and more than 120 genera of skippers. -

Phylogeny and Evolution of Lepidoptera
EN62CH15-Mitter ARI 5 November 2016 12:1 I Review in Advance first posted online V E W E on November 16, 2016. (Changes may R S still occur before final publication online and in print.) I E N C N A D V A Phylogeny and Evolution of Lepidoptera Charles Mitter,1,∗ Donald R. Davis,2 and Michael P. Cummings3 1Department of Entomology, University of Maryland, College Park, Maryland 20742; email: [email protected] 2Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560 3Laboratory of Molecular Evolution, Center for Bioinformatics and Computational Biology, University of Maryland, College Park, Maryland 20742 Annu. Rev. Entomol. 2017. 62:265–83 Keywords Annu. Rev. Entomol. 2017.62. Downloaded from www.annualreviews.org The Annual Review of Entomology is online at Hexapoda, insect, systematics, classification, butterfly, moth, molecular ento.annualreviews.org systematics This article’s doi: Access provided by University of Maryland - College Park on 11/20/16. For personal use only. 10.1146/annurev-ento-031616-035125 Abstract Copyright c 2017 by Annual Reviews. Until recently, deep-level phylogeny in Lepidoptera, the largest single ra- All rights reserved diation of plant-feeding insects, was very poorly understood. Over the past ∗ Corresponding author two decades, building on a preceding era of morphological cladistic stud- ies, molecular data have yielded robust initial estimates of relationships both within and among the ∼43 superfamilies, with unsolved problems now yield- ing to much larger data sets from high-throughput sequencing. Here we summarize progress on lepidopteran phylogeny since 1975, emphasizing the superfamily level, and discuss some resulting advances in our understanding of lepidopteran evolution. -

Amphiesmeno- Ptera: the Caddisflies and Lepidoptera
CY501-C13[548-606].qxd 2/16/05 12:17 AM Page 548 quark11 27B:CY501:Chapters:Chapter-13: 13Amphiesmeno-Amphiesmenoptera: The ptera:Caddisflies The and Lepidoptera With very few exceptions the life histories of the orders Tri- from Old English traveling cadice men, who pinned bits of choptera (caddisflies)Caddisflies and Lepidoptera (moths and butter- cloth to their and coats to advertise their fabrics. A few species flies) are extremely different; the former have aquatic larvae, actually have terrestrial larvae, but even these are relegated to and the latter nearly always have terrestrial, plant-feeding wet leaf litter, so many defining features of the order concern caterpillars. Nonetheless, the close relationship of these two larval adaptations for an almost wholly aquatic lifestyle (Wig- orders hasLepidoptera essentially never been disputed and is supported gins, 1977, 1996). For example, larvae are apneustic (without by strong morphological (Kristensen, 1975, 1991), molecular spiracles) and respire through a thin, permeable cuticle, (Wheeler et al., 2001; Whiting, 2002), and paleontological evi- some of which have filamentous abdominal gills that are sim- dence. Synapomorphies linking these two orders include het- ple or intricately branched (Figure 13.3). Antennae and the erogametic females; a pair of glands on sternite V (found in tentorium of larvae are reduced, though functional signifi- Trichoptera and in basal moths); dense, long setae on the cance of these features is unknown. Larvae do not have pro- wing membrane (which are modified into scales in Lepi- legs on most abdominal segments, save for a pair of anal pro- doptera); forewing with the anal veins looping up to form a legs that have sclerotized hooks for anchoring the larva in its double “Y” configuration; larva with a fused hypopharynx case. -

Influence of Habitat and Bat Activity on Moth Community Composition and Seasonal Phenology Across Habitat Types
INFLUENCE OF HABITAT AND BAT ACTIVITY ON MOTH COMMUNITY COMPOSITION AND SEASONAL PHENOLOGY ACROSS HABITAT TYPES BY MATTHEW SAFFORD THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology in the Graduate College of the University of Illinois at Urbana-Champaign, 2018 Urbana, Illinois Advisor: Assistant Professor Alexandra Harmon-Threatt, Chair and Director of Research ABSTRACT Understanding the factors that influence moth diversity and abundance is important for monitoring moth biodiversity and developing conservation strategies. Studies of moth habitat use have primarily focused on access to host plants used by specific moth species. How vegetation structure influences moth communities within and between habitats and mediates the activity of insectivorous bats is understudied. Previous research into the impact of bat activity on moths has primarily focused on interactions in a single habitat type or a single moth species of interest, leaving a large knowledge gap on how habitat structure and bat activity influence the composition of moth communities across habitat types. I conducted monthly surveys at sites in two habitat types, restoration prairie and forest. Moths were collected using black light bucket traps and identified to species. Bat echolocation calls were recorded using ultrasonic detectors and classified into phonic groups to understand how moth community responds to the presence of these predators. Plant diversity and habitat structure variables, including tree diameter at breast height, ground cover, and vegetation height were measured during summer surveys to document how differences in habitat structure between and within habitats influences moth diversity. I found that moth communities vary significantly between habitat types. -
Home Pre-Fire Moth Species List by Species
Species present before fire - by species Scientific Name Common Name Family Abantiades aphenges Hepialidae Abantiades hyalinatus Mustard Ghost Moth Hepialidae Abantiades labyrinthicus Hepialidae Acanthodela erythrosema Oecophoridae Acantholena siccella Oecophoridae Acatapaustus leucospila Nolidae Achyra affinitalis Cotton Web Spinner Crambidae Aeolochroma mniaria Geometridae Ageletha hemiteles Oecophoridae Aglaosoma variegata Notodontidae Agriophara discobola Depressariidae Agrotis munda Brown Cutworm Noctuidae Alapadna pauropis Erebidae Alophosoma emmelopis Erebidae Amata nigriceps Erebidae Amelora demistis Pointed Cape Moth Geometridae Amelora sp. Cape Moths Geometridae Antasia flavicapitata Geometridae Anthela acuta Common Anthelid Moth Anthelidae Anthela ferruginosa Anthelidae Anthela repleta Anthelidae Anthela sp. Anthelidae Anthela varia Variable Anthelid Anthelidae Antipterna sp. Oecophoridae Ardozyga mesochra Gelechiidae Ardozyga sp. Gelechiidae Ardozyga xuthias Gelechiidae Arhodia lasiocamparia Pink Arhodia Geometridae Arrade destituta Erebidae Arrade leucocosmalis Erebidae Asthenoptycha iriodes Tortricidae Asura lydia Erebidae Azelina biplaga Geometridae Barea codrella Oecophoridae Calathusa basicunea Nolidae Calathusa hypotherma Nolidae Capusa graodes Geometridae Capusa sp. Geometridae Carposina sp. Carposinidae Casbia farinalis Geometridae Casbia sp. Geometridae Casbia tanaoctena Geometridae Catacometes phanozona Oecophoridae Catoryctis subparallela Xyloryctidae Cernia amyclaria Geometridae Chaetolopha oxyntis Geometridae Chelepteryx -

Phylogenomics Reveals Major Diversification Rate Shifts in The
bioRxiv preprint doi: https://doi.org/10.1101/517995; this version posted January 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Phylogenomics reveals major diversification rate shifts in the evolution of silk moths and 2 relatives 3 4 Hamilton CA1,2*, St Laurent RA1, Dexter, K1, Kitching IJ3, Breinholt JW1,4, Zwick A5, Timmermans 5 MJTN6, Barber JR7, Kawahara AY1* 6 7 Institutional Affiliations: 8 1Florida Museum of Natural History, University of Florida, Gainesville, FL 32611 USA 9 2Department of Entomology, Plant Pathology, & Nematology, University of Idaho, Moscow, ID 10 83844 USA 11 3Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK 12 4RAPiD Genomics, 747 SW 2nd Avenue #314, Gainesville, FL 32601. USA 13 5Australian National Insect Collection, CSIRO, Clunies Ross St, Acton, ACT 2601, Canberra, 14 Australia 15 6Department of Natural Sciences, Middlesex University, The Burroughs, London NW4 4BT, UK 16 7Department of Biological Sciences, Boise State University, Boise, ID 83725, USA 17 *Correspondence: [email protected] (CAH) or [email protected] (AYK) 18 19 20 Abstract 21 The silkmoths and their relatives (Bombycoidea) are an ecologically and taxonomically 22 diverse superfamily that includes some of the most charismatic species of all the Lepidoptera. 23 Despite displaying some of the most spectacular forms and ecological traits among insects, 24 relatively little attention has been given to understanding their evolution and the drivers of 25 their diversity. -
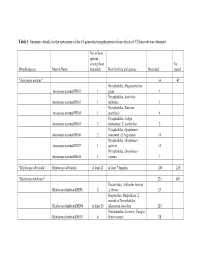
Table 1: Summary Details for the Specimens of the 16 Generalist Morphospecies from Which a COI Barcode Was Obtained
Table 1: Summary details for the specimens of the 16 generalist morphospecies from which a COI barcode was obtained No. of host species among those No. Morphospecies Interim Name barcoded Host families and species Barcoded reared "Anoxynops auratus" 34 49 Nymphalidae, Magneuptychia Anoxynops auratusDHJ01 1 gigas 1 Nymphalidae, Antirrhea Anoxynops auratusDHJ03 1 miltiades 1 Nymphalidae, Siderone Anoxynops auratusDHJ04 1 marthesia 4 Nymphalidae, Caligo Anoxynops auratusDHJ05 2 telamonius, C. eurilochus 2 Nymphalidae, Opsiphanes Anoxynops auratusDHJ06 2 tamarindi, O. bogotanus 10 Nymphalidae, Opsiphanes Anoxynops auratusDHJ07 1 quiteria 10 Nymphalidae, Opsiphanes Anoxynops auratusDHJ08 1 cassina 7 "Blepharipa albicauda" Blepharipa albicauda at least 21 at least 7 families 108 226 "Blepharipa fimbriata" 221 483 Hesperiidae, Achlyodes busirus, Blepharipa fimbriataDHJ01 2 A. thraso 23 Hesperiidae, Hesperiinae; 2 records of Nymphalidae, Blepharipa fimbriataDHJ04 at least 24 Manataria maculata 123 Notodontidae, Lirimiris, Farigia, Blepharipa fimbriataDHJ11 4 Heterocampa 28 Saturniidae, Syssphinx, Blepharipa fimbriataDHJ12 4 Ptiloscola dargei 47 "Chetogena scutellaris" 82 172 Chetogena scutellarisDHJ01 at least 22 at least 12 families 38 Chetogena scutellarisDHJ02 at least 20 at least 9 families 44 "Drino piceiventris" 223 455 Sphingidae, Callionima Drino piceiventrisDHJ03 1 denticulata 22 Drino piceiventrisDHJ04 1 Sphingidae, Adhemarius ypsilon 13 Sphingidae, Manduca, Erinnyis, Drino piceiventrisDHJ05 12 Eumorpha, Madoryx; rain forest 46 Sphingidae, Manduca, Protambulyx, Madoryx, dry Drino piceiventrisDHJ06 6 forest 16 Sphingidae, Enyo, Eumorpha, Aleuron, on Drino piceiventrisDHJ07 5 Dilleniaceae/Vitaceae 27 Sphingidae, Cautethia spuria, C. Drino piceiventrisDHJ08 2 yucatana 4 Sphingidae, Erinnyis, Drino piceiventrisDHJ09 3 Eupyrrhoglossum 4 Sphingidae, Enyo, Aleuron, Drino piceiventrisDHJ10 5 Erinnyis 14 Drino piceiventrisDHJ11 3 Sphinigdae, Pachylia, Erinnyis 24 Drino piceiventrisDHJ13 2 Sphingidae, Enyo 5 Sphingidae, Aellopos fadus, A. -

Impacts of Native and Non-Native Plants on Urban Insect Communities: Are Native Plants Better Than Non-Natives?
Impacts of Native and Non-native plants on Urban Insect Communities: Are Native Plants Better than Non-natives? by Carl Scott Clem A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Master of Science Auburn, Alabama December 12, 2015 Key Words: native plants, non-native plants, caterpillars, natural enemies, associational interactions, congeneric plants Copyright 2015 by Carl Scott Clem Approved by David Held, Chair, Associate Professor: Department of Entomology and Plant Pathology Charles Ray, Research Fellow: Department of Entomology and Plant Pathology Debbie Folkerts, Assistant Professor: Department of Biological Sciences Robert Boyd, Professor: Department of Biological Sciences Abstract With continued suburban expansion in the southeastern United States, it is increasingly important to understand urbanization and its impacts on sustainability and natural ecosystems. Expansion of suburbia is often coupled with replacement of native plants by alien ornamental plants such as crepe myrtle, Bradford pear, and Japanese maple. Two projects were conducted for this thesis. The purpose of the first project (Chapter 2) was to conduct an analysis of existing larval Lepidoptera and Symphyta hostplant records in the southeastern United States, comparing their species richness on common native and alien woody plants. We found that, in most cases, native plants support more species of eruciform larvae compared to aliens. Alien congener plant species (those in the same genus as native species) supported more species of larvae than alien, non-congeners. Most of the larvae that feed on alien plants are generalist species. However, most of the specialist species feeding on alien plants use congeners of native plants, providing evidence of a spillover, or false spillover, effect. -

Redalyc.The Skeleton and Musculature of Male and Female Terminalia in Oenosandra Boisduvalii Newman, 1856 and the Phylogenetic P
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Kuznetzov, V. I.; Naumann, C. M.; Speidel, W.; Stekolnikov, A. A. The skeleton and musculature of male and female terminalia in Oenosandra boisduvalii Newman, 1856 and the phylogenetic position of the family Oenosandridae (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 32, núm. 128, diciembre, 2004, pp. 297-313 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45512810 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 297 Kuznetzov (4-10-04) 3/1/77 18:27 Página 297 SHILAP Revta. lepid., 32 (128), 2004: 297-313 SRLPEF ISSN:0300-5267 The skeleton and musculature of male and female terminalia in Oenosandra boisduvalii Newman, 1856 and the phylogenetic position of the family Oenosandridae (Insecta: Lepidoptera) V. I. Kuznetzov, C. M. Naumann (†), W. Speidel & A. A. Stekolnikov Abstract The skeleton and musculature of the male and female terminalia have been studied in Oenosandra boisduvalii Newman, 1856, type species of the family Oenosandridae. It has been discovered that a comparatively high number of muscles of the male and female terminalia are included in the ground plan of ditrysian Lepidoptera. These are muscles m1, m3(2), m4, m5(7), m6(5), m7(6), m8(3), and m21. In the male genitalia only m2 (retractors of the anal cone) and m1-m7 and m10 in female terminalia have not been found.