Recent Advances in Endothelin Research on Cardiovascular and Endocrine Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differential Gene Expression Profile in Endometrioid And
[CANCER RESEARCH 63, 5697–5702, September 15, 2003] Advances in Brief Differential Gene Expression Profile in Endometrioid and Nonendometrioid Endometrial Carcinoma: STK15 Is Frequently Overexpressed and Amplified in Nonendometrioid Carcinomas1 Gema Moreno-Bueno, Carolina Sa´nchez-Este´vez, Rau´l Cassia, Sandra Rodrı´guez-Perales, Ramo´n Dı´az-Uriarte, Orlando Domı´nguez, David Hardisson, Miguel Andujar, Jaime Prat, Xavier Matias-Guiu, Juan C. Cigudosa, and Jose´Palacios2 Laboratory of Breast and Gynaecological Cancer, Molecular Pathology Programme [G. M-B., C. S-E., R. C., J. Pa.] and Biotechnology Programme [S. R-P., R. D-U., O. D., J. C. C.], Centro Nacional de Investigaciones Oncologicas, Madrid; Department of Pathology, Hospital Universitario La Paz, Madrid [D. H.]; Department of Pathology, Hospital Materno Infantil, Las Palmas [M. A.]; Department of Pathology, Hospital Sant Pau y Sant Creu, Barcelona [J. Pr.]; and Department of Pathology, Hospital Arnau de Villanova, Lleida [X. M-G.], Spain Abstract tiated endometrioid carcinomas that usually develop in pre- and perimenopausal women. They are associated with estrogen stimula- Endometrial carcinoma (EC) comprises at least two types of cancer: tion, coexist with, or are preceded by atypical endometrial hyperplasia endometrioid carcinomas (EECs) are estrogen-related tumors, which are and are associated with ER positivity and with K-RAS, PTEN, and frequently euploid and have a good prognosis. Nonendometrioid carcino-  mas (NEECs; serous and clear cell forms) are not estrogen related, are -catenin mutations, and microsatellite instability. Conversely, type II frequently aneuploid, and are clinically aggressive. We used cDNA mi- tumors are NEECs (papillary serous and clear cell carcinomas) that croarrays containing 6386 different genes to analyze gene expression occur in older women. -

The Cardiopulmonary Effects of the Calcitonin Gene- Related Peptide Family Kalsitonin-Geni İle İlişkili Peptit Ailesinin Kardiyopulmoner Etkileri
Turk J Pharm Sci 2020;17(3):349-356 DOI: 10.4274/tjps.galenos.2019.47123 REVIEW The Cardiopulmonary Effects of the Calcitonin Gene- related Peptide Family Kalsitonin-Geni İle İlişkili Peptit Ailesinin Kardiyopulmoner Etkileri Gökçen TELLİ1, Banu Cahide TEL1, Bülent GÜMÜŞEL2* 1Hacettepe University Faculty of Pharmacy, Department of Pharmacology, Ankara, Turkey 2Lokman Hekim University Faculty of Pharmacy, Department of Pharmacology, Ankara, Turkey ABSTRACT Cardiopulmonary diseases are very common among the population. They are high-cost diseases and there are still no definitive treatments. The roles of members of the calcitonin-gene related-peptide (CGRP) family in treating cardiopulmonary diseases have been studied for many years and promising results obtained. Especially in recent years, two important members of the family, adrenomedullin and adrenomedullin2/intermedin, have been considered new treatment targets in cardiopulmonary diseases. In this review, the roles of CGRP family members in cardiopulmonary diseases are investigated based on the studies performed to date. Key words: CGRP family, cardiopulmonary diseases, adrenomedullin, adrenomedullin2/intermedin, pulmonary hypertension ÖZ Kardiyopulmoner hastalıklar toplumda sık görülen, tedavi maliyeti oldukça yüksek ve halen kesin bir tedavisi bulunmayan hastalıklardır. Kalsitonin- geni ile ilişkili peptit (CGRP) ailesinin üyelerinin bir çok kardiyopulmoner hastalıktaki rolleri uzun yıllardır çalışılmakta ve umut vadeden sonuçlar elde edilmektedir. Özellikle son yıllarda CGRP ailesine -

Supporting Online Material
1 2 3 4 5 6 7 Supplementary Information for 8 9 Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic 10 retinopathy 11 12 *Samuel A. Mills, *Andrew I. Jobling, *Michael A. Dixon, Bang V. Bui, Kirstan A. Vessey, Joanna A. Phipps, 13 Ursula Greferath, Gene Venables, Vickie H.Y. Wong, Connie H.Y. Wong, Zheng He, Flora Hui, James C. 14 Young, Josh Tonc, Elena Ivanova, Botir T. Sagdullaev, Erica L. Fletcher 15 * Joint first authors 16 17 Corresponding author: 18 Prof. Erica L. Fletcher. Department of Anatomy & Neuroscience. The University of Melbourne, Grattan St, 19 Parkville 3010, Victoria, Australia. 20 Email: [email protected] ; Tel: +61-3-8344-3218; Fax: +61-3-9347-5219 21 22 This PDF file includes: 23 24 Supplementary text 25 Figures S1 to S10 26 Tables S1 to S7 27 Legends for Movies S1 to S2 28 SI References 29 30 Other supplementary materials for this manuscript include the following: 31 32 Movies S1 to S2 33 34 35 36 1 1 Supplementary Information Text 2 Materials and Methods 3 Microglial process movement on retinal vessels 4 Dark agouti rats were anaesthetized, injected intraperitoneally with rhodamine B (Sigma-Aldrich) to label blood 5 vessels and retinal explants established as described in the main text. Retinal microglia were labelled with Iba-1 6 and imaging performed on an inverted confocal microscope (Leica SP5). Baseline images were taken for 10 7 minutes, followed by the addition of PBS (10 minutes) and then either fractalkine or fractalkine + candesartan 8 (10 minutes) using concentrations outlined in the main text. -

Searching for Novel Peptide Hormones in the Human Genome Olivier Mirabeau
Searching for novel peptide hormones in the human genome Olivier Mirabeau To cite this version: Olivier Mirabeau. Searching for novel peptide hormones in the human genome. Life Sciences [q-bio]. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00340710 HAL Id: tel-00340710 https://tel.archives-ouvertes.fr/tel-00340710 Submitted on 21 Nov 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC THESE pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Discipline : Biologie Informatique Ecole Doctorale : Sciences chimiques et biologiques pour la santé Formation doctorale : Biologie-Santé Recherche de nouvelles hormones peptidiques codées par le génome humain par Olivier Mirabeau présentée et soutenue publiquement le 30 janvier 2008 JURY M. Hubert Vaudry Rapporteur M. Jean-Philippe Vert Rapporteur Mme Nadia Rosenthal Examinatrice M. Jean Martinez Président M. Olivier Gascuel Directeur M. Cornelius Gross Examinateur Résumé Résumé Cette thèse porte sur la découverte de gènes humains non caractérisés codant pour des précurseurs à hormones peptidiques. Les hormones peptidiques (PH) ont un rôle important dans la plupart des processus physiologiques du corps humain. -

EDNRB Gene Endothelin Receptor Type B
EDNRB gene endothelin receptor type B Normal Function The EDNRB gene provides instructions for making a protein called endothelin receptor type B. This protein is located on the surface of cells and functions as a signaling mechanism, transmitting information from outside the cell to inside the cell. The receptor interacts with proteins called endothelins to regulate several critical biological processes, including the development and function of blood vessels, the production of certain hormones, and the stimulation of cell growth and division. Endothelin 3 (produced from the EDN3 gene) is one of the proteins that interacts with endothelin receptor type B. During early development before birth (embryonic development), endothelin 3 and endothelin receptor type B together play an important role in neural crest cells. These cells migrate from the developing spinal cord to specific regions in the embryo, where they give rise to many different types of cells. In particular, endothelin 3 and endothelin receptor type B are essential for the formation of nerves in the intestine (enteric nerves) and for the production of specialized cells called melanocytes. Melanocytes produce melanin, a pigment that contributes to skin, hair, and eye color. Melanin is also involved in the normal function of the inner ear. Health Conditions Related to Genetic Changes Hirschsprung disease More than 30 mutations in the EDNRB gene have been found to cause Hirschsprung disease, a disorder that causes severe constipation or blockage of the intestine. Although Hirschsprung disease is a feature of another condition called Waardenburg syndrome type IV (described below), EDNRB gene mutations can also cause Hirschsprung disease in people without Waardenburg syndrome. -
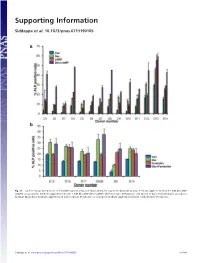
Supporting Information
Supporting Information Siddappa et al. 10.1073/pnas.0711190105 Fig. S1. (a) Percentage ALP-positive cells in hMSCs grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with 1 mM db-cAMP (cAMP), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). (b) Percentage ALP-positive cells grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with forskolin (Forskolin), or osteogenic medium supplemented with forskolin (DexϩForskolin). Siddappa et al. www.pnas.org/cgi/content/short/0711190105 1of10 Fig. S2. hMSCs were grown in basic medium, basic medium supplemented with 1 mM db-cAMP (cAMP), osteogenic medium (Dex), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). Expression was analyzed by qPCR and is expressed as fold induction compared with cells grown in basic medium. The data were analyzed by using two-way ANOVA, and statistical significance is indicated compared with cells grown in basic medium. *, P Ͻ 0.05. Siddappa et al. www.pnas.org/cgi/content/short/0711190105 2of10 Fig. S3. (a) Methylene blue staining of hMSC-seeded scaffolds grown in basic medium (Con) or basic medium supplemented with 1 mM db-cAMP (cAMP) for 4 days. Note the less intensely stained db-cAMP-treated construct, indicating reduced cell numbers. (b) Quantitative Alamar blue assay for cell number analysis. The data were analyzed by using one-way ANOVA followed by Dunnet’s multiple-comparison test. Statistical significance is indicated compared with cells grown in basic medium (Con). *, P Ͻ 0.05 Siddappa et al. www.pnas.org/cgi/content/short/0711190105 3of10 Fig. -
![A Genomic Atlas of Human Adrenal and Gonad Development [Version 2; Referees: 4 Approved] Ignacio Del Valle1, Federica Buonocore1, Andrew J](https://docslib.b-cdn.net/cover/1314/a-genomic-atlas-of-human-adrenal-and-gonad-development-version-2-referees-4-approved-ignacio-del-valle1-federica-buonocore1-andrew-j-711314.webp)
A Genomic Atlas of Human Adrenal and Gonad Development [Version 2; Referees: 4 Approved] Ignacio Del Valle1, Federica Buonocore1, Andrew J
Wellcome Open Research 2017, 2:25 Last updated: 08 NOV 2017 RESEARCH ARTICLE A genomic atlas of human adrenal and gonad development [version 2; referees: 4 approved] Ignacio del Valle1, Federica Buonocore1, Andrew J. Duncan1, Lin Lin1, Martino Barenco2, Rahul Parnaik1, Sonia Shah3,4, Mike Hubank5, Dianne Gerrelli2, John C. Achermann 1 1Genetics and Genomic Medicine, UCL Great Ormond Street Institute of Child Health, London, UK 2Developmental Biology and Cancer, UCL Great Ormond Street Institute of Child Health, London, UK 3Institute for Molecular Bioscience, University of Queensland, Brisbane, Australia 4Institute of Cardiovascular Science, University College London, London, UK 5The Centre for Molecular Pathology, Royal Marsden Hospital, Sutton, UK v2 First published: 07 Apr 2017, 2:25 (doi: 10.12688/wellcomeopenres.11253.1) Open Peer Review Latest published: 23 Oct 2017, 2:25 (doi: 10.12688/wellcomeopenres.11253.2) Referee Status: Abstract Background: In humans, the adrenal glands and gonads undergo distinct biological events between 6-10 weeks post conception (wpc), such as testis Invited Referees determination, the onset of steroidogenesis and primordial germ cell 1 2 3 4 development. However, relatively little is currently known about the genetic mechanisms underlying these processes. We therefore aimed to generate a detailed genomic atlas of adrenal and gonad development across these critical version 2 report report stages of human embryonic and fetal development. published Methods: RNA was extracted from 53 tissue samples between 6-10 wpc 23 Oct 2017 (adrenal, testis, ovary and control). Affymetrix array analysis was performed and differential gene expression was analysed using Bioconductor. A version 1 mathematical model was constructed to investigate time-series changes across published report report report report 07 Apr 2017 the dataset. -

Table of Contents
Therapeutic systems for Insulin-like growth factor-I Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Julius-Maximilians-Universität Würzburg vorgelegt von Isabel Schultz aus Dahn Würzburg 2015 Eingereicht bei der Fakultät für Chemie und Pharmazie am Gutachter der schriftlichen Arbeit 1. Gutachter: 2. Gutachter: Prüfer des öffentlichen Promotionskolloquiums 1. Prüfer: 2. Prüfer: 3. Prüfer: Datum des öffentlichen Promotionskolloquiums Doktorurkunde ausgehändigt am TABLE OF CONTENTS TABLE OF CONTENTS SUMMARY ............................................................................... 1 ZUSAMMEMFASSUNG .......................................................... 5 CHAPTER I ............................................................................... 9 DRUG DELIVERY OF INSULIN-LIKE GROWTH FACTOR I CHAPTER II ............................................................................ 45 INSULIN-LIKE GROWTH FACTOR-I AEROSOL FORMULATIONS FOR PULMONARY DELIVERY CHAPTER III ........................................................................... 73 PULMONARY INSULIN-LIKE GROWTH FACTOR I DELIVERY FROM TREHALOSE AND SILK-FIBROIN MICROPARTICLES CHAPTER IV ......................................................................... 113 EXPRESSION OF IGF-I MUTANTS CONCLUSION AND OUTLOOK ......................................... 147 DOCUMENTATION OF AUTHORSHIP ............................. 159 CURRICULUM VITAE ......................................................... 163 ACKNOWLEDGMENTS ..................................................... -

Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile
Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile Julien Patenaude and Claude Perreault This information is current as J Immunol 2016; 196:4760-4770; Prepublished online 29 of October 1, 2021. April 2016; doi: 10.4049/jimmunol.1502499 http://www.jimmunol.org/content/196/11/4760 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2016/04/29/jimmunol.150249 Material 9.DCSupplemental References This article cites 65 articles, 18 of which you can access for free at: http://www.jimmunol.org/content/196/11/4760.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on October 1, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile Julien Patenaude and Claude Perreault In order to understand the role of mesenchymal cells (MCs) in the adult thymus, we performed whole transcriptome analyses of primary thymic, bone, and skin MCs. -

Growth Hormone and Insulin-Like Growth Factors I and II Produce
Proc. Natl. Acad. Sci. USA Vol. 82, pp. 8724-8728, December 1985 Medical Sciences Growth hormone and insulin-like growth factors I and II produce distinct alterations in glucose metabolism in 3T3-F442A adipocytes (glucose oxidation/lipid accumulation/somatomedin C/multiplication-stimulating activity/recombinant DNA-derived insulin-like growth factor I) JESSICA SCHWARTZ, CAROL M. FOSTER, AND MARTA S. SATIN Department of Physiology, University of Michigan Medical School, Ann Arbor, MI 48109 Communicated by H. W. Davenport, August 20, 1985 ABSTRACT In 3T3-F442A adipocytes, human growth difficult (9, 10) because the onset of the suppressive effects hormone (hGH) stimulates glucose oxidation in 4 hr. A requires several days and in vitro preparations sensitive to maximal increase is evident at hGH concentrations of 50-100 GH (e.g., adipocytes) are not viable for extended periods. ng/ml and rarely exceeds 50% above control. The stimulation Recently, we reported that cultured 3T3-F442A adipocytes is transient; after 48 hr of incubation with GH, glucose could be used to study the metabolic effects of GH (11). The oxidation is significantly suppressed to 35% below control differentiation of 3T3-F442A fibroblasts to adipocytes has values. In view of the concept that insulin-like growth factors been shown to be GH-dependent (12), although GH is not (IGF) may mediate the effects of GH, we compared the effects required to maintain the adipocytes. We have found that, ofhGH (500 ng/ml) and several preparations ofIGF on glucose once converted, the 3T3 adipocytes are sensitive to both metabolism in 3T3 adipocytes. After 4 hr of incubation, IGF-I stimulatory and suppressive effects of human GH (hGH) on from human plasma stimulated glucose oxidation in a dose- glucose metabolism (11). -

Nitric Oxide As a Second Messenger in Parathyroid Hormone-Related Protein Signaling
433 Nitric oxide as a second messenger in parathyroid hormone-related protein signaling L Kalinowski, L W Dobrucki and T Malinski Department of Chemistry and Biochemistry, Ohio University, Athens, Ohio, USA (Requests for offprints should be addressed to T Malinski, Department of Chemistry and Biochemistry, Ohio University, Biochemistry Research Laboratories 136, Athens, Ohio 45701–2979, USA; Email: [email protected]) (L Kalinowski was on sabbatical leave from the Department of Clinical Biochemistry, Medical University of Gdansk and Laboratory of Cellular and Molecular Nephrology, Medical Research Center of the Polish Academy of Science, Poland) Abstract Parathyroid hormone (PTH)-related protein (PTHrP) is competitive PTH/PTHrP receptor antagonists, 10 µmol/l produced in smooth muscles and endothelial cells and [Leu11,-Trp12]-hPTHrP(7–34)amide and 10 µmol/l is believed to participate in the local regulation of vascu- [Nle8,18,Tyr34]-bPTH(3–34)amide, were equipotent in lar tone. No direct evidence for the activation of antagonizing hPTH(1–34)-stimulated NO release; endothelium-derived nitric oxide (NO) signaling pathway [Leu11,-Trp12]-hPTHrP(7–34)amide was more potent by PTHrP has been found despite attempts to identify it. than [Nle8,18,Tyr34]-bPTH(3–34)amide in inhibiting Based on direct in situ measurements, it is reported here for hPTHrP(1–34)-stimulated NO release. The PKC inhibi- the first time that the human PTH/PTHrP receptor tor, H-7 (50 µmol/l), did not change hPTH(1–34)- and analogs, hPTH(1–34) and hPTHrP(1–34), stimulate NO hPTHrP(1–34)-stimulated NO release, whereas the release from a single endothelial cell. -

EDN3 Gene Endothelin 3
EDN3 gene endothelin 3 Normal Function The EDN3 gene provides instructions for making a protein called endothelin 3. Proteins in the endothelin family are produced in various cells and tissues, where they are involved in the development and function of blood vessels, the production of certain hormones, and the stimulation of cell growth and division. Endothelin 3 functions by interacting with another protein, endothelin receptor type B ( produced from the EDNRB gene), on the surface of cells. During early development before birth, endothelin 3 and endothelin receptor type B together play an important role in neural crest cells. These cells migrate from the developing spinal cord to specific regions in the embryo, where they give rise to many different types of cells. In particular, endothelin 3 and its receptor are essential for the formation of nerves in the intestine ( enteric nerves) and for the production of specialized cells called melanocytes. Melanocytes produce melanin, a pigment that contributes to skin, hair, and eye color. Melanin is also involved in the normal function of the inner ear. Health Conditions Related to Genetic Changes Hirschsprung disease About 10 mutations in the EDN3 gene have been found to cause Hirschsprung disease, a disorder that causes severe constipation or blockage of the intestine. Although Hirschsprung disease is a feature of another disorder called Waardenburg syndrome type IV (described below), EDN3 gene mutations can also cause Hirschsprung disease in people without Waardenburg syndrome. These mutations change one DNA building block (nucleotide) or insert an additional nucleotide in the gene. Changes in the EDN3 gene disrupt the normal function of endothelin 3, preventing it from playing its usual role in the development of enteric nerves.