Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FLRT Proteins Are Endogenous Latrophilin Ligands and Regulate Excitatory Synapse Development
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Neuron Report FLRT Proteins Are Endogenous Latrophilin Ligands and Regulate Excitatory Synapse Development Matthew L. O’Sullivan,1,5 Joris de Wit,1,5 Jeffrey N. Savas,2 Davide Comoletti,3 Stefanie Otto-Hitt,1,6 John R. Yates III,2 and Anirvan Ghosh1,4,* 1Neurobiology Section, Division of Biology, University of California San Diego, La Jolla, CA 92093, USA 2Department of Chemical Physiology, The Scripps Research Institute, La Jolla, CA 92037, USA 3Child Health Institute of New Jersey and Department of Neuroscience and Cell Biology, UMDNJ/Robert Wood Johnson Medical School, New Brunswick, NJ 08901, USA 4CNS Discovery, F. Hoffmann-La Roche, 4070 Basel, Switzerland 5These authors contributed equally to this work 6Present address: Department of Natural Sciences, Carroll College, Helena, MT 59625, USA *Correspondence: [email protected] DOI 10.1016/j.neuron.2012.01.018 SUMMARY much effort has been expended investigating the mechanisms of a-latrotoxin action (Su¨ dhof, 2001), nothing is known about Latrophilins (LPHNs) are a small family of G protein- the endogenous function of latrophilins in vertebrates. Further coupled receptors known to mediate the massive evidence for the importance of latrophilins in the proper synaptic exocytosis caused by the black widow functioning of neural circuits comes from recent human spider venom a-latrotoxin, but their endogenous genetics studies that have linked LPHN3 mutations to attention ligands and function remain unclear. Mutations in deficit hyperactivity disorder (ADHD), a common and highly LPHN3 are strongly associated with attention deficit heritable developmental psychiatric disorder (Arcos-Burgos et al., 2010; Domene´ et al., 2011; Jain et al., 2011; Ribase´ s hyperactivity disorder, suggesting a role for latrophi- et al., 2011). -

Edinburgh Research Explorer
Edinburgh Research Explorer International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list Citation for published version: Davenport, AP, Alexander, SPH, Sharman, JL, Pawson, AJ, Benson, HE, Monaghan, AE, Liew, WC, Mpamhanga, CP, Bonner, TI, Neubig, RR, Pin, JP, Spedding, M & Harmar, AJ 2013, 'International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands', Pharmacological reviews, vol. 65, no. 3, pp. 967-86. https://doi.org/10.1124/pr.112.007179 Digital Object Identifier (DOI): 10.1124/pr.112.007179 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Pharmacological reviews Publisher Rights Statement: U.S. Government work not protected by U.S. copyright General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 1521-0081/65/3/967–986$25.00 http://dx.doi.org/10.1124/pr.112.007179 PHARMACOLOGICAL REVIEWS Pharmacol Rev 65:967–986, July 2013 U.S. -
Supporting Information
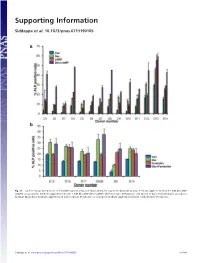
Supporting Information Siddappa et al. 10.1073/pnas.0711190105 Fig. S1. (a) Percentage ALP-positive cells in hMSCs grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with 1 mM db-cAMP (cAMP), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). (b) Percentage ALP-positive cells grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with forskolin (Forskolin), or osteogenic medium supplemented with forskolin (DexϩForskolin). Siddappa et al. www.pnas.org/cgi/content/short/0711190105 1of10 Fig. S2. hMSCs were grown in basic medium, basic medium supplemented with 1 mM db-cAMP (cAMP), osteogenic medium (Dex), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). Expression was analyzed by qPCR and is expressed as fold induction compared with cells grown in basic medium. The data were analyzed by using two-way ANOVA, and statistical significance is indicated compared with cells grown in basic medium. *, P Ͻ 0.05. Siddappa et al. www.pnas.org/cgi/content/short/0711190105 2of10 Fig. S3. (a) Methylene blue staining of hMSC-seeded scaffolds grown in basic medium (Con) or basic medium supplemented with 1 mM db-cAMP (cAMP) for 4 days. Note the less intensely stained db-cAMP-treated construct, indicating reduced cell numbers. (b) Quantitative Alamar blue assay for cell number analysis. The data were analyzed by using one-way ANOVA followed by Dunnet’s multiple-comparison test. Statistical significance is indicated compared with cells grown in basic medium (Con). *, P Ͻ 0.05 Siddappa et al. www.pnas.org/cgi/content/short/0711190105 3of10 Fig. -

Table of Contents
Therapeutic systems for Insulin-like growth factor-I Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Julius-Maximilians-Universität Würzburg vorgelegt von Isabel Schultz aus Dahn Würzburg 2015 Eingereicht bei der Fakultät für Chemie und Pharmazie am Gutachter der schriftlichen Arbeit 1. Gutachter: 2. Gutachter: Prüfer des öffentlichen Promotionskolloquiums 1. Prüfer: 2. Prüfer: 3. Prüfer: Datum des öffentlichen Promotionskolloquiums Doktorurkunde ausgehändigt am TABLE OF CONTENTS TABLE OF CONTENTS SUMMARY ............................................................................... 1 ZUSAMMEMFASSUNG .......................................................... 5 CHAPTER I ............................................................................... 9 DRUG DELIVERY OF INSULIN-LIKE GROWTH FACTOR I CHAPTER II ............................................................................ 45 INSULIN-LIKE GROWTH FACTOR-I AEROSOL FORMULATIONS FOR PULMONARY DELIVERY CHAPTER III ........................................................................... 73 PULMONARY INSULIN-LIKE GROWTH FACTOR I DELIVERY FROM TREHALOSE AND SILK-FIBROIN MICROPARTICLES CHAPTER IV ......................................................................... 113 EXPRESSION OF IGF-I MUTANTS CONCLUSION AND OUTLOOK ......................................... 147 DOCUMENTATION OF AUTHORSHIP ............................. 159 CURRICULUM VITAE ......................................................... 163 ACKNOWLEDGMENTS ..................................................... -

The Roles of Fgfs in the Early Development of Vertebrate Limbs
Downloaded from genesdev.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW The roles of FGFs in the early development of vertebrate limbs Gail R. Martin1 Department of Anatomy and Program in Developmental Biology, School of Medicine, University of California at San Francisco, San Francisco, California 94143–0452 USA ‘‘Fibroblast growth factor’’ (FGF) was first identified 25 tion of two closely related proteins—acidic FGF and ba- years ago as a mitogenic activity in pituitary extracts sic FGF (now designated FGF1 and FGF2, respectively). (Armelin 1973; Gospodarowicz 1974). This modest ob- With the advent of gene isolation techniques it became servation subsequently led to the identification of a large apparent that the Fgf1 and Fgf2 genes are members of a family of proteins that affect cell proliferation, differen- large family, now known to be comprised of at least 17 tiation, survival, and motility (for review, see Basilico genes, Fgf1–Fgf17, in mammals (see Coulier et al. 1997; and Moscatelli 1992; Baird 1994). Recently, evidence has McWhirter et al. 1997; Hoshikawa et al. 1998; Miyake been accumulating that specific members of the FGF 1998). At least five of these genes are expressed in the family function as key intercellular signaling molecules developing limb (see Table 1). The proteins encoded by in embryogenesis (for review, see Goldfarb 1996). Indeed, the 17 different FGF genes range from 155 to 268 amino it may be no exaggeration to say that, in conjunction acid residues in length, and each contains a conserved with the members of a small number of other signaling ‘‘core’’ sequence of ∼120 amino acids that confers a com- molecule families [including WNT (Parr and McMahon mon tertiary structure and the ability to bind heparin or 1994), Hedgehog (HH) (Hammerschmidt et al. -

Growth Hormone and Insulin-Like Growth Factors I and II Produce
Proc. Natl. Acad. Sci. USA Vol. 82, pp. 8724-8728, December 1985 Medical Sciences Growth hormone and insulin-like growth factors I and II produce distinct alterations in glucose metabolism in 3T3-F442A adipocytes (glucose oxidation/lipid accumulation/somatomedin C/multiplication-stimulating activity/recombinant DNA-derived insulin-like growth factor I) JESSICA SCHWARTZ, CAROL M. FOSTER, AND MARTA S. SATIN Department of Physiology, University of Michigan Medical School, Ann Arbor, MI 48109 Communicated by H. W. Davenport, August 20, 1985 ABSTRACT In 3T3-F442A adipocytes, human growth difficult (9, 10) because the onset of the suppressive effects hormone (hGH) stimulates glucose oxidation in 4 hr. A requires several days and in vitro preparations sensitive to maximal increase is evident at hGH concentrations of 50-100 GH (e.g., adipocytes) are not viable for extended periods. ng/ml and rarely exceeds 50% above control. The stimulation Recently, we reported that cultured 3T3-F442A adipocytes is transient; after 48 hr of incubation with GH, glucose could be used to study the metabolic effects of GH (11). The oxidation is significantly suppressed to 35% below control differentiation of 3T3-F442A fibroblasts to adipocytes has values. In view of the concept that insulin-like growth factors been shown to be GH-dependent (12), although GH is not (IGF) may mediate the effects of GH, we compared the effects required to maintain the adipocytes. We have found that, ofhGH (500 ng/ml) and several preparations ofIGF on glucose once converted, the 3T3 adipocytes are sensitive to both metabolism in 3T3 adipocytes. After 4 hr of incubation, IGF-I stimulatory and suppressive effects of human GH (hGH) on from human plasma stimulated glucose oxidation in a dose- glucose metabolism (11). -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

Comparison of the Interactions of Different Growth Factors And
molecules Article Comparison of the Interactions of Different Growth Factors and Glycosaminoglycans Fuming Zhang 1,* , Lanhong Zheng 2,3, Shuihong Cheng 2,4, Yanfei Peng 2,5, Li Fu 2, Xing Zhang 2,6 and Robert J. Linhardt 1,2,7,* 1 Department of Chemical and Biological Engineering, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA 2 Department of Chemistry and Chemical Biology, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA; [email protected] (L.Z.); [email protected] (S.C.); [email protected] (Y.P.); [email protected] (L.F.); [email protected] (X.Z.) 3 School of Pharmacy, Shanghai University of Medicine & Health Sciences, Shanghai 201318, China 4 CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China 5 College of Marine Life Sciences, Ocean University of China, 5 Yushan Road, Qingdao 266003, China 6 School of Food Science and Pharmaceutical Engineering, Nanjing Normal University, Wenyuan Road 1, Nanjing 210023, China 7 Departments of Biology and Biomedical Engineering, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA * Correspondence: [email protected] (F.Z.); [email protected] (R.J.L.); Tel.: +1-518-276-3404 (R.J.L.) Received: 20 August 2019; Accepted: 11 September 2019; Published: 16 September 2019 Abstract: Most growth factors are naturally occurring proteins, which are signaling molecules implicated in cellular multiple functions such as proliferation, migration and differentiation under patho/physiological conditions by interacting with cell surface receptors and other ligands in the extracellular microenvironment. -

Recent Advances in Endothelin Research on Cardiovascular and Endocrine Systems
Endocrine Journal 1994, 41(5), 491-507 Review Recent Advances in Endothelin Research on Cardiovascular and Endocrine Systems MITSUHIDE NARUSE, KIYoxo NARUSE, AND HIR0SH1 DEMURA Department of Medicine, Institute of Clinical Endocrinology, TokyoWomen's Medical College, Tokyo162, Japan Introduction Structure of ET and ET Receptor Since the quite exciting discovery in 1988 of The molecular and biochemical aspects of ET endothelin (ET) in the culture medium of aortic en- family peptides and ET receptors have been re- dothelial cells by Yanagisawa and coworkers [1], viewed by Masaki and Yanagisawa [13] and others evidence has accumulated to support its important [14-16]. We here describe the fundamentals. roles in the regulation of blood pressure and body fluid homeostasis and its pathophysiological sig- 1. ET Peptides nificance in various cardiovascular diseases. The discovery of ET and the opponent substances in- The ET molecule consists of 21 amino acid resi- cluding nitric oxide [2] and natriuretic peptides [3] dues with two intramolecular disulfide bonds be- during the last decade was epoch-making in the tween cystein residues at 1 and 15 and 3 and 11, field of "Cardiovascular Endocrinology". respectively. The ring structure and the hydropho- Because of its impressive potency and the long bic amino acid residues at the C-terminus are in- duration of its pressor action [1], the roles of ET in dispensable to its full biological activities. There the regulation of cardiovascular homeostasis have are three distinct isof orms of ET: ET-1, ET-2, and been extensively studied. However, the co-expres- ET-3 [13]. The difference in the amino acid se- sion or close localization of ET and its receptors in quence is seen in the ring structure, while they various tissues other than the vascular vessels sug- share the same sequence in the linear C-terminal gest non-vascular roles of ET [4-6]. -

An Investig Atio N of the Anabolic
AN INVESTIGATION OF THE ANABOLIC ACTIONS OF BIOSYNTHETIC HUMAN GROWTH HORMONE AFTER INJURY BY BURNING A thesis sumitted for the degree of MASTER OF SURGERY in the UNIVERSITY OF LONDON H.J.C.R. Belcher, MB, BS(Lond), FRCS(Eng). The Blond-Mclndoe Centre, Queen Victoria Hospital, Holtye Rd, East Grinstead, SUSSEX. - 1 - ProQuest Number: U053257 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest U053257 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 To my wife, Georgina - 2 - ABSTRACT Previous clinical trials in normal subjects and post-operative patients have shown that biosynthetic growth hormone preparations increase nitrogen retention. It has been suggested that their administration to injured patients may be beneficial. A clinical trial is presented of twelve adult burned patients of whom six were allocated to receive biosynthetic human growth hormone (somatropin) and six to form a control group. Injury by burning is followed by increases in resting energy expenditure and urinary nitrogen excretion, accompanied by insulin resistance and glucose intolerance. There is a generalised fall in plasma protein concentrations, including the somatomedin, insulin-like growth factor-I. -

New Structural Perspectives in G Protein-Coupled Receptor-Mediated Src Family Kinase Activation
International Journal of Molecular Sciences Review New Structural Perspectives in G Protein-Coupled Receptor-Mediated Src Family Kinase Activation Sandra Berndt * and Ines Liebscher Rudolf Schönheimer Institute of Biochemistry, Molecular Biochemistry, Medical Faculty, University of Leipzig, 04103 Leipzig, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-341-9722175 Abstract: Src family kinases (SFKs) are key regulators of cell proliferation, differentiation, and survival. The expression of these non-receptor tyrosine kinases is strongly correlated with cancer development and tumor progression. Thus, this family of proteins serves as an attractive drug target. The activation of SFKs can occur via multiple signaling pathways, yet many of them are poorly understood. Here, we summarize the current knowledge on G protein-coupled receptor (GPCR)- mediated regulation of SFKs, which is of considerable interest because GPCRs are among the most widely used pharmaceutical targets. This type of activation can occur through a direct interaction between the two proteins or be allosterically regulated by arrestins and G proteins. We postulate that a rearrangement of binding motifs within the active conformation of arrestin-3 mediates Src regulation by comparison of available crystal structures. Therefore, we hypothesize a potentially different activation mechanism compared to arrestin-2. Furthermore, we discuss the probable direct regulation of SFK by GPCRs and investigate the intracellular domains of exemplary GPCRs with conserved polyproline binding motifs that might serve as scaffolding domains to allow such a direct interaction. Large intracellular domains in GPCRs are often understudied and, in general, not much Citation: Berndt, S.; Liebscher, I. is known of their contribution to different signaling pathways. -

Fgf10 Dosage Is Critical for the Amplification of Epithelial Cell Progenitors and for the Formation of Multiple Mesenchymal Lineages During Lung Development
Developmental Biology 307 (2007) 237–247 www.elsevier.com/locate/ydbio Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development Suresh K. Ramasamy a,1, Arnaud A. Mailleux b,1, Varsha V. Gupte a, Francisca Mata a, Frédéric G. Sala a, Jacqueline M. Veltmaat c, Pierre M. Del Moral a, Stijn De Langhe a, Sara Parsa a, Lisa K. Kelly d, Robert Kelly e, Wei Shia a, Eli Keshet f, Parviz Minoo g, ⁎ David Warburton a, Savério Bellusci a, a Developmental Biology Program, Saban Research Institute of Childrens Hospital Los Angeles, Los Angeles, CA 90027, USA b Department of Cell Biology, Harvard Medical School, Boston, MA 02115, USA c Institute of Molecular and Cell Biology, 61 Biopolis Drive, Proteos, Singapore d Division of Pediatrics, Childrens Hospital Los Angeles, Los Angeles, CA 90027, USA e Developmental Biology Institute of Marseille Luminy-UMR6216-CNRS-Université de la Méditerranée, France f Department of Molecular Biology, The Hebrew University–Hadassah Medical School, Jerusalem, Israel g Department of Pediatrics, Women's and Children's Hospital, USC Keck School of Medicine, Los Angeles, CA 90033, USA Received for publication 23 October 2006; revised 24 April 2007; accepted 26 April 2007 Available online 3 May 2007 Abstract The key role played by Fgf10 during early lung development is clearly illustrated in Fgf10 knockout mice, which exhibit lung agenesis. However, Fgf10 is continuously expressed throughout lung development suggesting extended as well as additional roles for FGF10 at later stages of lung organogenesis. We previously reported that the enhancer trap Mlcv1v-nLacZ-24 transgenic mouse strain functions as a reporter for Fgf10 expression and displays decreased endogenous Fgf10 expression.